Non-melanoma skin cancers (NMSC) are the most common type of skin malignancies among humans (particularly fair-skinned populations of European descent) and its incidence rates have been on the rise globally for decades [1]. The best defined role of vitamin D in humans is in supporting the normal development and maintenance of bone tissues and in regulating calcium metabolism [7,8]. Furthermore, there is growing evidence that vitamin D plays a role in many fundamental biological processes (e.g., cell proliferation, angiogenesis, and modulation of the immune system) [9] implicated in carcinogenesis.
1. Introduction
Non-melanoma skin cancers (NMSC) are the most common type of skin malignancies among humans (particularly fair-skinned populations of European descent) and its incidence rates have been on the rise globally for decades [1]. The near totality of NMSC is represented by keratinocyte skin cancers (KSC), e.g., basal cell cancer (BCC) and squamous cell cancer (SCC), while other non-melanoma skin cancer types not originating from keratinocytes (e.g., Merkel cell carcinoma) are rare. The economic costs required by the management of NMSC patients are substantial because of very high NMSC incidence rates [2]. The most important environmental risk factor for NMSC is exposure of the skin to ultraviolet radiation (UV) [3]. Other established and suspected risk factors include older age, male sex, light-coloured skin, eyes and hair, use of photosensitizing medications, and having had a previous NMSC diagnosis [4][5]. Given the high NMSC disease burden, research has largely focused on identifying other preventable risk factors, and several publications have examined the role of vitamin D in the aetiology of NMSC.
Vitamin D is produced in human skin and is also found naturally in some foods [6]. In addition, vitamin D-fortified foods are available on the market, and vitamin D can be obtained by taking supplements. In the body, vitamin D is hydroxylated first in the liver to form 25-hydroxivitamin D [25(OH)D], which is the major circulating form of vitamin D, and then in the kidney to form the physiologically active 1,25-dihydroxyvitamin D. Most vitamin D in the blood is bound to the vitamin D binding protein (VDBP). To exert its action, calcitriol binds to the vitamin D receptor (VDR): several polymorphisms of the VDR gene lead to an altered functionality of the VDR protein, and have been investigated in association with the occurrence of several diseases.
The best defined role of vitamin D in humans is in supporting the normal development and maintenance of bone tissues and in regulating calcium metabolism [7][8]. Furthermore, there is growing evidence that vitamin D plays a role in many fundamental biological processes (e.g., cell proliferation, angiogenesis, and modulation of the immune system) [9] implicated in carcinogenesis.
2. Vitamin D and Non-Melanoma Skin Cancer
2.1. Vitamin D Blood Concentration and NMSC Risk
Non-melanoma skin cancers (NMSC) are the most common type of skin malignancies among humans (particularly fair-skinned populations of European descent) and its incidence rates have been on the rise globally for decades [1]. The near totality of NMSC is represented by keratinocyte skin cancers (KSC), e.g., basal cell cancer (BCC) and squamous cell cancer (SCC), while other non-melanoma skin cancer types not originating from keratinocytes (e.g., Merkel cell carcinoma) are rare. The economic costs required by the management of NMSC patients are substantial because of very high NMSC incidence rates [2]. The most important environmental risk factor for NMSC is exposure of the skin to ultraviolet radiation (UV) [3]. Other established and suspected risk factors include older age, male sex, light-coloured skin, eyes and hair, use of photosensitizing medications, and having had a previous NMSC diagnosis [4,5]. Given the high NMSC disease burden, research has largely focused on identifying other preventable risk factors, and several publications have examined the role of vitamin D in the aetiology of NMSC.
Vitamin D is produced in human skin and is also found naturally in some foods [6]. In addition, vitamin D-fortified foods are available on the market, and vitamin D can be obtained by taking supplements. In the body, vitamin D is hydroxylated first in the liver to form 25-hydroxivitamin D [25(OH)D], which is the major circulating form of vitamin D, and then in the kidney to form the physiologically active 1,25-dihydroxyvitamin D. Most vitamin D in the blood is bound to the vitamin D binding protein (VDBP). To exert its action, calcitriol binds to the vitamin D receptor (VDR): several polymorphisms of the VDR gene lead to an altered functionality of the VDR protein, and have been investigated in association with the occurrence of several diseases.
The best defined role of vitamin D in humans is in supporting the normal development and maintenance of bone tissues and in regulating calcium metabolism [7,8]. Furthermore, there is growing evidence that vitamin D plays a role in many fundamental biological processes (e.g., cell proliferation, angiogenesis, and modulation of the immune system) [9] implicated in carcinogenesis.
2. Vitamin D and Non-Melanoma Skin Cancer
2.1. Vitamin D Blood Concentration and NMSC Risk
Ten studies reported a RR estimate comparing NMSC risk among those in the highest vs. lowest category of serum/plasma 25(OH)D concentration (Table 1) [10][11][12][13][14][15][16][17][18][19][10,11,12,13,14,15,16,17,18,19]. Of these, five were conducted in the USA, two in Denmark, and one each in Australia, Brazil, and Poland. In terms of study design, three were case-control studies [13][17][19][13,17,19], two were nested case-control studies [10][11][10,11], and five were cohort studies [12][14][15][16][18][12,14,15,16,18]. The ten studies encompassed a total of 3899 NMSC cases, of which 1569 (40.2%) were contributed by Winsløw et al. [18]. Vitamin D concentration was measured in serum in all studies except in Liang et al. [14]. The studies differed greatly both in the categories that were used to calculate the RR for the highest vs. lowest vitamin D concentration comparison, and in the degree of statistical adjustment. In particular, for three studies an unadjusted OR was calculated using data provided in the paper [13][17][19][13,17,19].
2.2. Vitamin D Dietary Intake and Supplements Use and NMSC Risk
Five studies reported on the association between vitamin D intake (from diet, from supplements, or both) and NMSC risk [24,25,26,27,28] ( Table 1. Main characteristics of the studies reporting on the association between serum/plasma concentration of 25(OH)D (comparison: highest vs. lowest category) and the risk of non-melanoma skin cancer.
2.2. Vitamin D Dietary Intake and Supplements Use and NMSC Risk
Five studies reported on the association between vitamin D intake (from diet, from supplements, or both) and NMSC risk [20][21][22][23][24] (Table 2). Davies et al. reported a case-control study of 109 BCC cases and 247 controls nested in a population-based UK cohort and found no significant association between vitamin D intake from food and BCC risk [20]. Likewise, no significant association emerged in the population-based case-control study by Asgari et al., which included 415 SCC and an equal number of controls in the USA [21]. Park et al. analyzed data from the Nurses’ Health and Health Professionals Follow-up prospective studies and reported an increased risk of BCC (but not SCC) among those in the highest quintile of total vitamin D intake (food + supplements) [22]. Finally, in two RCT, both conducted in the USA, NMSC risk was compared among study participants being given vitamin D supplements and those in the placebo group, but no significant association was found [23][24]. We did not calculate a SRR because of the heterogeneity across studies in terms of study design (two RCTS and three observational studies) and type of exposure (vitamin D from food, supplements, or both).
Table 2. Main characteristics of the studies reporting on the association between vitamin D intake (from foods, supplements, or both) and the risk of non-melanoma skin cancer.
| Author, Year |
Country |
Study Design |
Skin Cancer Type |
N Cases |
N Controls/Cohort Size |
% Males |
Age at NMSC (yrs) |
Years of Diagnosis |
Exposure |
Comparison |
RR |
95% CI |
Adjusting Variables |
| Davies, 2002 [20] |
UK |
]. Park et al. analyzed data from the Nurses’ Health and Health Professionals Follow-up prospective studies and reported an increased risk of BCC (but not SCC) among those in the highest quintile of total vitamin D intake (food + supplements) [26]. Finally, in two RCT, both conducted in the USA, NMSC risk was compared among study participants being given vitamin D supplements and those in the placebo group, but no significant association was found [27,28]. We did not calculate a SRR because of the heterogeneity across studies in terms of study design (two RCTS and three observational studies) and type of exposure (vitamin D from food, supplements, or both).
2.3. VDR and VDBP Genes Polymorphisms and NMSC Risk
Five papers reported on the association between any of five polymorphisms of the VDR gene (Apa1, Bsm1, Cdx2, Fok1, and Taq1) and NMSC risk [13,29,30,31,32]. The studies were conducted in the USA (n = 2) and Europe (n = 3) ( Table 4. Main characteristics of the studies reporting on the association between polymorphisms of the vitamin D receptor (VDR) gene and the risk of non-melanoma skin cancer.
| Author, Year |
Country |
Study Design |
Skin Cancer Type |
N Cases |
N Controls |
VDR Polymorphisms |
| Apa1 |
Fok1 |
Bsm1 |
Cdx2 |
Taq1 |
| NCC |
BCC |
109 |
| Han, 2007 [30] |
USA |
NCC |
BCC |
295 | 247 |
52.3% |
mean 66, range 46–79 |
1993–1998 |
intake from food |
linear increase by 2.08 microg/d |
1.07 |
0.84–1.35 |
age, sex, phenotype, other |
| 853 |
|
x |
x |
x |
|
Asgari, 2011 [21] |
USA |
CC |
SCC |
415 |
415 |
61.9% |
mean 72.5, range 43–85 |
2004 |
supplement use ≥3 months in the past 10 years |
any vs. none |
0.78 |
0.46–1.32 |
| SCC | age, sex, phenotype, other |
| 281 |
|
x |
x |
x |
|
Tang, 2011 [22] (a) |
USA |
RCT |
NMSC |
3338 |
36,282 |
0.0% |
ns |
1995 |
200 IU twice daily (intervention arm) |
supplementation vs. placebo |
1.02 |
0.95–1.07 |
age |
| Lesiak, 2011 [13] |
Poland |
hCC |
BCC |
142 |
142 |
x |
x |
x |
|
x |
Park, 2016 [23] (b) |
USA |
cohort |
BCC |
20,840 |
109,290 |
38.0% |
ns |
1984–2010 |
intake from food + supplements |
5th vs. 1st quintile |
1.10 |
1.05–1.15 |
age, sex, phenotype, phototype, UV exposure, other |
| Köstner, 2012 [31] |
Germany |
hCC |
BCC |
87 |
50 |
x |
|
SCC |
2329 |
1.02 |
0.89–1.17 |
| Passarelli, 2020 |
USA |
RCT |
BCC |
200 |
2259 |
63.0% |
ns |
2004–2016 |
1000 IU/day (intervention arm) |
supplementation vs. placebo |
0.96 |
0.73–1.26 |
age, sex, UV exposure, other |
| SCC |
68 |
0.79 |
0.49–1.27 |
(a) Participants in the intervention arm received 500 mg of elemental Ca twice daily in addition to vitamin D. (b) Results were also available for vitamin D from foods only, and stratified for the two sub-cohorts (Nurses’ Health Study and Health Professionals Follow-up Study).
2.3. VDR and VDBP Genes Polymorphisms and NMSC Risk
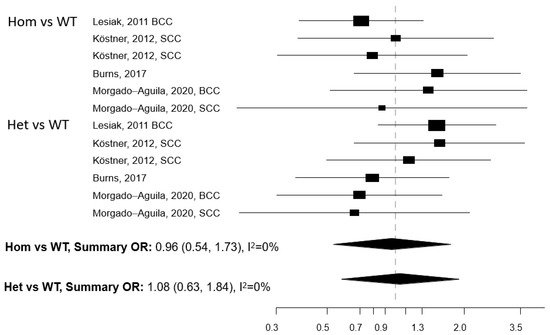
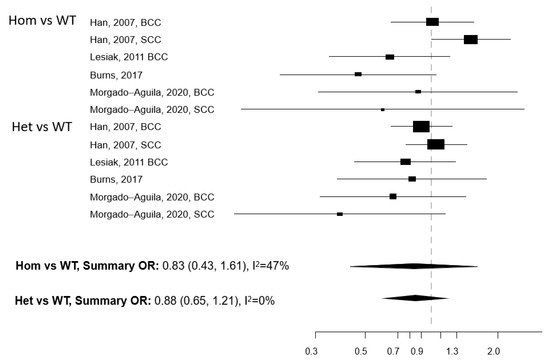
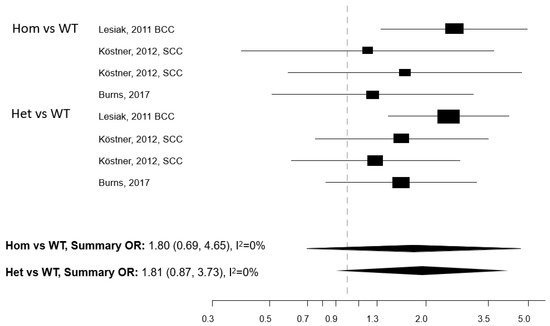
Five papers reported on the association between any of five polymorphisms of the VDR gene (Apa1, Bsm1, Cdx2, Fok1, and Taq1) and NMSC risk [13][25][26][27][28]. The studies were conducted in the USA (n = 2) and Europe (n = 3) (Table 3). Meta-analysis was conducted for three polymorphisms: Apa1, Bsm1, and Taq1: no association with NMSC risk emerged for any of these three polymorphisms, either in the Hom vs. WT or in the Het vs. WT models (Figure 1, Figure 2 and Figure 3). The heterogeneity was below 50% for all models. The relationship between Cdx2 and Fok1 polymorphism and NMSC risk was examined in the paper by Han et al. [26]: no significant association emerged in any of the models that were fitted. Instead, the Fok1 TT (Hom) genotype was reported to significantly increase BCC risk (OR = 10.14, p-value < 0.001) in the study by Lesiak et al. [13].
). Davies et al. reported a case-control study of 109 BCC cases and 247 controls nested in a population-based UK cohort and found no significant association between vitamin D intake from food and BCC risk [24]. Likewise, no significant association emerged in the population-based case-control study by Asgari et al., which included 415 SCC and an equal number of controls in the USA [25
| |
| x |
| SCC |
100 |
x |
|
|
|
x |
| Burns, 2017 [29] |
USA |
hCC |
KSC |
97 |
100 |
x |
|
x |
|
x |
| Morgado-Águila, 2020 [32] |
Spain |
hCC |
BCC |
61 |
73 |
x |
|
x |
|
|
| SCC |
20 |
x |
|
x |
|
|
| Author, Year |
Country |
Study Design |
Skin Cancer Type |
N Cases |
N Controls/Cohort Size |
% Males |
Age at NMSC (yrs) |
Years of Diagnosis |
Exposure |
Comparison |
RR |
95% CI |
Adjusting Variables |
| Asgari, 2010 [10] |
USA |
NCC |
BCC |
220 |
220 |
51.8% |
mean 54.9, range 28–78 |
1968–1989 |
serum 25(OH)D |
5th vs. 1st quintile (>29.8 vs. <14.7 ng/mL) |
2.09 |
0.95–4.58 |
age, sex, season of sampling, phenotype, UV exposure, other |
| Skelsey, 2010 [19] (a) (b) |
USA |
CC |
KSC |
50 |
14 |
ns |
range 18–65 |
ns |
serum 25(OH)D |
≥30 vs. <30 ng/ml |
0.16 |
0.004–1.30 |
none (c) |
| Tang, 2010 [11] |
USA |
NCC |
NMSC |
178 |
930 |
100.0% |
mean 73.6 |
2000–2007 |
serum 25(OH)D |
5th vs. 1st quintile (≥29.9 vs. <16 ng/mL) |
0.54 |
0.31–0.96 |
age, season of sampling, other |
| Eide, 2011 [12] |
USA |
cohort |
KSC |
240 |
3223 |
10.7% |
ns |
1997–2009 |
serum 25(OH)D |
4th vs. 1st quartile (≥31 vs. <19 ng/mL) |
1.6 |
1.1–2.3 |
age, sex |
| Lesiak, 2011 [13] |
Poland |
CC |
BCC |
142 |
142 |
50.0% |
mean 56, range 45–78 |
2007–2008 |
serum 25(OH)D |
>30 vs. <20 ng/ml |
0.18 |
0.08–0.37 |
none (c) |
| Liang, 2012 [14] |
USA |
cohort |
BCC |
510 |
4641 |
0.0% |
ns |
1976–2008 |
plasma 25(OH)D |
4th vs. 1st quartile |
2.07 |
1.52–2.80 |
age, season of sampling, UV exposure, phenotype, phototype, other |
| SCC |
75 |
3.77 |
1.70–8.36 |
| van der Pols, 2013 [15] |
Australia |
cohort |
BCC |
300 |
1191 |
50.0% |
mean 58 |
1996–2007 |
serum 25(OH)D |
≥75 vs. <75 nmol/L |
1.51 |
1.10–2.07 |
age, sex, UV exposure, phenotype, phototype, other |
| SCC |
176 |
56.0% |
mean 63 |
≥75 vs. <75 nmol/L |
0.67 |
0.44–1.03 |
| Skaaby, 2014 [16] |
Denmark |
cohort |
NMSC |
398 |
12,204 |
48.1% |
ns |
1993–2011 |
serum 25(OH)D |
4th vs. 1st quartile |
1.43 |
1.05–1.93 |
age, sex, season of sampling, other |
| Soares, 2018 [17] (b) |
Brazil |
CC |
KSC |
41 |
200 |
56.1% |
mean 67, range 21–87 |
2016–2017 |
serum 25(OH)D |
≥30 vs. <20 ng/ml |
50.00 |
11.11–100.0 |
none (c) |
| Winsløw, 2018 [18] |
Denmark |
cohort |
NMSC |
1569 |
35,298 |
43.0% |
ns |
1981–2012 |
plasma 25(OH)D |
≥50 vs. <25 nmol/L |
3.76 |
2.58–5.48 |
age, sex, season of sampling, other |
CC: case-control. NCC: nested case-control. BCC: basal cell cancer. SCC: squamous cell cancer. KSC: keratinocyte skin cancer. NMSC: non-melanoma skin cancer. (a) Conference abstract. (b) RR were inverted (compared to what reported in the text) so that the category of patients with lowest 25(OH)d concentration is the category of reference. (c) Unadjusted OR calculated using data provided in the contingency table.
). Meta-analysis was conducted for three polymorphisms: Apa1, Bsm1, and Taq1: no association with NMSC risk emerged for any of these three polymorphisms, either in the Hom vs. WT or in the Het vs. WT models (
Figure 1. Forest plot for the association between the Apa1 polymorphism of the vitamin D receptor (VDR) gene and the risk of non-melanoma skin cancer. BCC: basal cell cancer. SCC: squamous cell cancer. RR: relative risk. Hom: homozygous. Het: heterozygous. WT: wild-type.
Figure 2. Forest plot for the association between the Bsm1 polymorphism of the vitamin D receptor (VDR) gene and the risk of non-melanoma skin cancer. BCC: basal cell cancer. SCC: squamous cell cancer. RR: relative risk. Hom: homozygous. Het: heterozygous. WT: wild-type.
Figure 3. Forest plot for the association between the Taq1 polymorphism of the vitamin D receptor (VDR) gene and the risk of non-melanoma skin cancer. BCC: basal cell cancer. SCC: squamous cell cancer. RR: relative risk. Hom: homozygous. Het: heterozygous. WT: wild-type.
5). The heterogeneity was below 50% for all models. The relationship between Cdx2 and Fok1 polymorphism and NMSC risk was examined in the paper by Han et al. [30]: no significant association emerged in any of the models that were fitted. Instead, the Fok1 TT (Hom) genotype was reported to significantly increase BCC risk (OR = 10.14, p-value < 0.001) in the study by Lesiak et al. [13].
A single study that considered polymorphisms in the VDBP gene and NMSC risk [29][33]. The study relied on 7983 participants, of which 235 developed BCC during follow-up. BCC was not associated with the two polymorphisms of the VDBP gene (rs7041 and rs4588) that were investigated, despite some limited evidence of an age-specific effect.
There was some evidence that individuals with higher measured plasma or serum 25(OH)D concentration were at increased NMSC risk. However, studies were greatly heterogeneous, which suggests caution in drawing conclusions, particularly regarding the magnitude of the possible association. Vitamin D intake was associated with a mild increase in BCC risk in the large observational study by Park et al.; however, this finding was not confirmed in another four studies, two of which had a RCT design. Finally, NMSC risk was not associated with any single polymorphism of the VDR or VDBP genes.
The association between serum/plasma 25(OH)D concentration and NMSC risk is most likely due to UV radiation exposure being causally linked to both vitamin D concentration in the blood and NMSC risk. The mild, yet significantly increased BCC risk observed among individuals with higher vitamin D intake in the large study by Park et al. is difficult to explain, particularly in light of the growing evidence in favour of a protective effect played by vitamin D supplementation against cancer at several body sites [30][31][32]. However, the finding by Park et al. was mild, limited to BCC, and not confirmed in any other study, including two vitamin D supplementation RCTs which, because of their experimental design, are expected to be less susceptible to biases (e.g., confounding and misclassification) affecting observational studies. By and large, a strong association between vitamin D intake or supplementation and NMSC risk seems unlikely, and vitamin D supplementation should continue to be considered as an effective and reasonably safe method of achieving the recommended amount of vitamin D.
Individuals carrying polymorphisms at the VDR or VDBP genes do not seem to suffer from an increased NMSC risk, with the possible exception of the VDR TaqI gene polymorphism. However, the number of studies eligible for inclusion in each gene polymorphism-specific meta-analysis model was limited, which prevents drawing firm conclusions. The studied polymorphisms of the VDR gene are known to impair the functionality of the receptor and eventually disrupt several vitamin D-linked biological pathways [8]. Considering that VDR polymorphisms may affect the risk of cancer at multiple body sites [33] and that an effect on NMSC risk cannot be ruled out a priori, we recommend that more studies are conducted in this research area.
There was some evidence that individuals with higher measured plasma or serum 25(OH)D concentration were at increased NMSC risk. However, studies were greatly heterogeneous, which suggests caution in drawing conclusions, particularly regarding the magnitude of the possible association. Vitamin D intake was associated with a mild increase in BCC risk in the large observational study by Park et al.; however, this finding was not confirmed in another four studies, two of which had a RCT design. Finally, NMSC risk was not associated with any single polymorphism of the VDR or VDBP genes.
The association between serum/plasma 25(OH)D concentration and NMSC risk is most likely due to UV radiation exposure being causally linked to both vitamin D concentration in the blood and NMSC risk. The mild, yet significantly increased BCC risk observed among individuals with higher vitamin D intake in the large study by Park et al. is difficult to explain, particularly in light of the growing evidence in favour of a protective effect played by vitamin D supplementation against cancer at several body sites [34,35,36]. However, the finding by Park et al. was mild, limited to BCC, and not confirmed in any other study, including two vitamin D supplementation RCTs which, because of their experimental design, are expected to be less susceptible to biases (e.g., confounding and misclassification) affecting observational studies. By and large, a strong association between vitamin D intake or supplementation and NMSC risk seems unlikely, and vitamin D supplementation should continue to be considered as an effective and reasonably safe method of achieving the recommended amount of vitamin D.
Individuals carrying polymorphisms at the VDR or VDBP genes do not seem to suffer from an increased NMSC risk, with the possible exception of the VDR TaqI gene polymorphism. However, the number of studies eligible for inclusion in each gene polymorphism-specific meta-analysis model was limited, which prevents drawing firm conclusions. The studied polymorphisms of the VDR gene are known to impair the functionality of the receptor and eventually disrupt several vitamin D-linked biological pathways [8]. Considering that VDR polymorphisms may affect the risk of cancer at multiple body sites [37] and that an effect on NMSC risk cannot be ruled out a priori, we recommend that more studies are conducted in this research area.
3. Conclusions
The link between vitamin D metabolism per se and NMSC risk is unlikely to exist, although some findings (in particular, the positive association between vitamin D intake from diet and supplements and BCC risk reported in a large observational study) are worthy of further investigation, for instance within existing large-scale RCTs including vitamin D supplementation as an experimental arm. The cornerstone of NMSC prevention must remain limiting exposure of the skin to UV light, and vitamin D supplementation may be recommended as the preferred method to secure the multiple health benefits of adequate vitamin D concentration (which extends far beyond the possible effects on the skin) while avoiding the health risks associated with an excessive exposure of the skin to the UV radiation.
The link between vitamin D metabolism per se and NMSC risk is unlikely to exist, although some findings (in particular, the positive association between vitamin D intake from diet and supplements and BCC risk reported in a large observational study) are worthy of further investigation, for instance within existing large-scale RCTs including vitamin D supplementation as an experimental arm. The cornerstone of NMSC prevention must remain limiting exposure of the skin to UV light, and vitamin D supplementation may be recommended as the preferred method to secure the multiple health benefits of adequate vitamin D concentration (which extends far beyond the possible effects on the skin) while avoiding the health risks associated with an excessive exposure of the skin to the UV radiation.