Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Dimitrina Zheleva-Dimitrova and Version 2 by Vivi Li.
The widespread genus Cirsium Mill. (thistle) is one of the biggest genera in Asteraceae family (subfamily: Carduoideae Cass. Ex Sweet, tribe: Cardueae Cass., subtribe: Carduinae (Cass.) Dumort, sect. Cirsium). It includes about 250 species spread throughout Europe, North Africa, East Asia, Central Asia, SW Asia and North and Central America. Its species have been used for many years as a traditional herbal medicine. As the origin of the name suggests (“khirsos” in Greek means “swollen veins”), the genus Cirsium has been known for centuries for its usage against varicose diseases, to relieve pain.
- Cirsium appendiculatum
- UHPLC–HRMS
- biochemometric
- antioxidants
- enzyme inhibitory activity
- partial least-square discriminant analysis
1. Introduction
Profiling methods for the analysis of crude plant extracts have evolved into powerful tools for dereplication, quality assessment and metabolomics. This procedure enables recognition of known metabolites at the earliest stage of separation, avoiding the time-consuming and expensive isolation of common constituents. The most current metabolite profiling studies are performed with state-of-the-art high-resolution LC–MS tools that apply the high resolution of ultra-high-performance liquid chromatography (UHPLC) for the chromatographic resolution of isomers, and high-resolution MS methods for molecular formula assignment [1]. In particular, the hybrid quadrupole-orbitrap has high mass resolution and accuracy in MS non-targeted profilings of specialized (secondary) natural products in crude extracts [2]. In this context, biochemometrics approaches, which rely on the use of statistical modelling tools to correlate metabolite profiles with biological datasets, are very useful for assigning biological activity to a particular compound detected from complex mixtures.
The widespread genus Cirsium Mill. (thistle) is one of the biggest genera in Asteraceae family (subfamily: Carduoideae Cass. Ex Sweet, tribe: Cardueae Cass., subtribe: Carduinae (Cass.) Dumort, sect. Cirsium). It includes about 250 species spread throughout Europe, North Africa, East Asia, Central Asia, SW Asia and North and Central America [3][4][3,4]. Its species have been used for many years as a traditional herbal medicine. As the origin of the name suggests (“khirsos” in Greek means “swollen veins”), the genus Cirsium has been known for centuries for its usage against varicose diseases, to relieve pain [4]. According to the ethnopharmacological relevance, the species is also valued for the treatment of numerous ailments due to its diuretic, astringent, anti-inflammatory, anti-melanogenesis, anti-tumor and anxiolitic activities as well as its activity against nonalcoholic fatty liver disease [5][6][5,6]. Additionally, some Cirsium species are used as a food source. Receptacles of C. spinosissimum have been traditionally eaten similarly to artichoke leaves by alpine populations [7]. Moreover, Cirsium species are valuable to the honey industry as they produce a good supply of nectar and pollen. In the past decade, invasive exotic species, such as Eurasian thistles, present a major threat to sustained productivity and biodiversity in the United States, and different Cirsium species have been assessed for biological control as weeds [8].
The taxon is characterized by the presence of a large number of secondary metabolites such as phenolic acids, flavonoids, sterols, triterpenes, alkaloids and lignans [8][9][8,9]. The existence of flavones, flavonols and flavonones, free aglycones, their derivatives and glycosides has been proven [8][10][11][8,10,11]. The best-known and researched compounds in Cirsium are the flavonoids, which are found in all plant organs. Acacetin, apigenin, cirsimaritin, luteolin, quercetin, pectolinarigenin and their glycosides are among the most common flavonoids [4][6][4,6].
Given the notable amount of data on the traditional medicinal usage and therapeutic properties attributed to the Cirsium species, it is necessary to scientifically bioprospect poorly studied species belonging to this genus, such as Cirsium appendiculatum Griseb. (Balkan thistle) against significant human diseases such as diabetes, Alzheimer’s disease, atherosclerosis, etc. C. appendiculatum is an herbaceous perennial plant, up to 180 cm height, flowering from June to September. It is a Balkan endemic plant occurring in Turkey, Greece, Albania, North Macedonia, Serbia and Montenegro [12]. The species is distributed in the alpine zone at altitudes between 1000–2500 m asl and occurs in a wide range of open habitats such as meadows, forests and rivers [12].
An investigation of its health-promoting effects and applications in a variety of nutraceutical, pharmaceutical, medicinal and cosmetic areas represents interest, and the data will complete knowledge of the genus. Accordingly, a wide range of biological activities such as antioxidant activity and inhibitory effects against different enzyme classes were investigated.
In line with the new paradigm in pharmacognosy to obtain massive metabolite profiling of natural extracts for a rational prioritization of bioactive natural products [13], the present study was designed to investigate, for the first time, the phytochemical profile and biological activity of C. appendiculatum extracts. An innovative/efficient workflow based on the association of both UHPLC–HRMS and biochemometrics, using a combination of multiple statistical models (partial least-square discriminant and heat-map analyses) to target bioactive compounds from extracts was developed.

2. Results and Discussion
The complete workflow combining UHPLC–HRMS with discriminant analysis of the chromatographic data and the biological potential is presented on Figure 1.
Figure 1. The complete workflow for the biochemometric approach. Samples (flower heads, aerial parts and root extracts) prepared at the same concentration are first injected into UHPLC–HRMS (A). Data are acquired using the data-dependent acquisition mode, then converted through MZmine 2 software processing. In parallel, spectrophotometric assays (B) and bioassays (C) are conducted to determine total phenolic and flavonoid contents and activity and information are tabulated. The final .csv files are then used for the generation of the biochemometic data by partial least-square discriminant analysis (PLS-DA) with R software. Finally, bioactivity mapping was performed.
2.1. UHPLC–HRMS Profiling of Specialized Natural Products in Cirsium appendiculatum Extracts
Based on retention times, MS and MS/MS accurate masses and relative ion abundance, elemental composition, fragmentation patterns in MS/MS spectra, conformity to the simulated monoisotopic profiles and comparison with reference standards and literature data, a total of 61 specialized natural products were identified or tentatively annotated in C. appendiculatum extracts (Table 1). The total ion chromatograms (TIC) of the studied extracts are depicted in Figure 2.
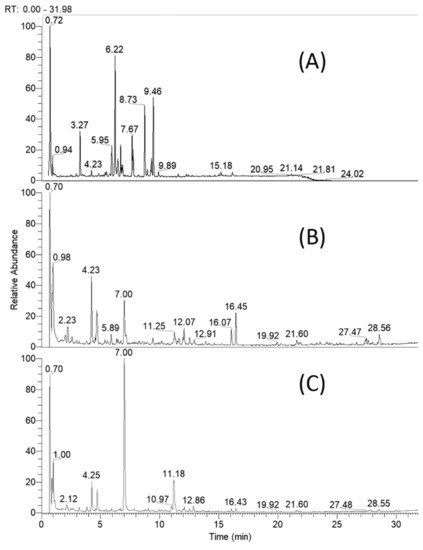

Figure 2. Total ion chromatogram of Cirsium appendiculatum extracts; (A) flower heads, (B) aerial parts, (C) roots.
Table 1. Specialized natural products in Cirsium appendiculatum extracts.
| № | Identified/Tentatively Annotated Compound | Molecular Formula | Exact Mass [M-H]− |
tR (Min) |
Δ ppm | Distribution | Level of Identification (CAWG) |
|---|---|---|---|---|---|---|---|
| Carboxylic (including hydroxybenzoic and hydroxycinnamic) acids | |||||||
| 1. | protocatechuic acid a | C7H6O4 | 153.0179 | 2.15 | −7.986 | 1, 2 | 1 |
| 2. | dihydroxybenzoic acid | C7H6O4 | 153.0181 | 3.44 | −8.182 | 2, 3 | 2 |
| 3. | gentisic acid a | C7H6O4 | 153.0179 | 3.86 | −9.685 | 2 | 1 |
| 4. | vanillic acid a | C8H8O4 | 167.0338 | 4.77 | −6.837 | 1, 2, 3 | 1 |
| 5. | caffeic acid a | C9H8O4 | 179.0340 | 3.51 | −5.317 | 1, 2, 3 | 1 |
| 6. | quinic acid | C7H12O6 | 191.0551 | 3.18 | −5.032 | 1, 2, 3 | 2 |
| 7. | eucomic acid | C11H12O6 | 239.0557 | 3.38 | −0.717 | 1, 2, 3 | 2 |
| 8. | caffeoyl-syringic acid | C18H16O8 | 359.0985 | 2.32 | 0.390 | 1, 2, 3 | 4 |
| Hydroxybenzoic and hydroxycinnamc acids glycosides | |||||||
| 9. | 4-hydroxyphenylacetic acid O-β-D-glucoside |
C14H18O8 | 313.0933 | 2.18 | 1.467 | 2 | 2 |
| 10. | vanillic acid O-deoxyhexoside | C14H18O8 | 313.0934 | 3.25 | 1.467 | 2, 3 | 2 |
| 11. | gentisic acid O-hexoside | C14H20O8 | 315.1087 | 1.92 | 0.601 | 1, 2, 3 | 2 |
| 12. | p-hydroxybenzoic acid O-hexoside | C14H20O8 | 315.1086 | 2.10 | 0.029 | 1, 2, 3 | 2 |
| 13. | vanillic acid O-hexoside | C14H18O9 | 329.0885 | 1.71 | 2.020 | 1, 2, 3 | 2 |
| 14. | leonuriside A | C14H20O9 | 331.1037 | 1.44 | 0.739 | 1, 2, 3 | 2 |
| 15. | gallic acid O-hexoside | C13H16O10 | 331.0676 | 1.58 | 1.601 | 2 | 2 |
| Acylquinic acids | |||||||
| 16. | 1-p-coumaroylquinic acid | C16H18O8 | 337.0932 | 4.61 | 1.007 | 1, 2 | 2 |
| 17. | 3-p-coumaroylquinic acid | C16H18O8 | 337.0935 | 3.01 | 1.748 | 2 | 2 |
| 18. | 1-caffeoylquinic acid | C16H18O9 | 353.0880 | 2.27 | 0.410 | 1, 2 | 2 |
| 19. | neochlorogenic (3-caffeoylquinic) acid | C16H18O9 | 353.0878 | 3.21 | −0.015 | 1, 2, 3 | 1 |
| 20. | chlorogenic (5-caffeoylquinic) acid a | C16H18O9 | 353.0874 | 3.94 | −1.233 | 1, 2, 3 | 1 |
| 21. | 4-caffeoylquinic acid | C16H18O9 | 353.0879 | 6.27 | 0.155 | 1, 2, 3 | 2 |
| 22. | 3,4-dicaffeoylquinic acid a | C25H24O12 | 515.1199 | 5.73 | 0.836 | 1, 2, 3 | 1 |
| 23. | 1,5-dicaffeoylquinic acid a | C25H24O12 | 515.1191 | 5.91 | −0.697 | 1, 2, 3 | 1 |
| 24. | 3,5-dicaffeoylquinic acid | C25H24O12 | 515.1199 | 6.08 | 0.720 | 1, 2, 3 | 1 |
| 25. | 4,5-dicaffeoylquinic acid | C25H24O12 | 515.1191 | 6.25 | −0.697 | 1, 2, 3 | 1 |
| 26. | 1,3,5-tricaffeoylquinic acid | C34H30O15 | 677.1512 | 5.15 | - | 1, 2, 3 | 1 |
| Flavonoids | |||||||
| 27. | apigenin a | C15H9O5 | 269.0459 | 8.58 | 1.313 | 1, 3 | 1 |
| 28. | genkwanin a | C16H12O5 | 283.0608 | 11.41 | −1.543 | 2 | 1 |
| 29. | acacetin | C16H12O5 | 283.0615 | 11.40 | 1.142 | 1, 3 | 2 |
| 30. | luteolin a | C15H10O6 | 285.0404 | 7.55 | −0.075 | 1, 3 | 1 |
| 31. | hispidulin (scutellarein-6-methyl ether) a | C16H12O6 | 299.0561 | 8. 81 | −0.172 | 1, 2, 3 | 1 |
| 32. | diosmetin | C16H12O6 | 299.0560 | 9.28 | −0.272 | 1 | 1 |
| 33. | quercetin a | C15H9O6 | 301.0354 | 7.61 | 1.11 | 1 | 1 |
| 34. | pectolinarigenin | C17H14O6 | 313.0722 | 12.26 | 1.305 | 1, 2, 3 | 2 |
| 35. | nepetin (6-methoxyluteolin) | C16H11O7 | 315.0514 | 8.09 | 1.251 | 1, 3 | 2 |
| 36. | cirsiliol | C17H14O7 | 329.0669 | 8.87 | 0.772 | 1 | 2 |
| 37. | apigenin 7-O-glucoside a | C21H20O10 | 431.0988 | 6.06 | 0.835 | 1 | 1 |
| 38. | kaempferol 3-O-deoxyhexoside | C21H20O10 | 431.0983 | 6.60 | −0.232 | 1, 2 | 2 |
| 39. | apigenin O-hexuronide | C21H18O11 | 445.0770 | 6.45 | −0.347 | 1, 2, 3 | 2 |
| 40. | kaempferol 3-O-glucoside a | C21H20O11 | 447.0935 | 5.63 | 0.571 | 1, 2 | 1 |
| 41. | luteolin 7-O-glucoside a | C21H19O11 | 447.0934 | 6.04 | 0.281 | 1, 2, 3 | 1 |
| 42. | luteolin 7-O-hexuronide | C21H18O12 | 461.0734 | 5.37 | 1.911 | 1, 3 | 2 |
| 43. | diosmetin 7-O-hexoside | C22H22O11 | 461.1092 | 6.30 | 0.684 | 1, 2, 3 | |
| 22 | O | 11 | 461.1093 | 6.67 | 0.966 | 1, 2, 3 | 2 |
| 45. | hispidulin-O-hexuronide | C22H20O12 | 475.0882 | 6.33 | 0.002 | 1, 3 | 2 |
| 46. | pectolinarigenin-O-hexoside | C23H24O11 | 475.1247 | 8.11 | 0.159 | 1 | 2 |
| 47. | nepetin-O-hexoside | C22H21O12 | 477.1040 | 5.65 | 0.316 | 1, 3 | 2 |
| 48. | nepetin-O-hexuronide | C22H20O13 | 491.0835 | 6.32 | 0.725 | 1 | 2 |
| 49. | acaciin (acacetin 7-O-rutinoside) a | C28H32O14 | 591.1730 | 7.59 | 3.622 | 1, 2, 3 | 1 |
| 50. | kaempferol 3-O-rutinoside a | C27H30O15 | 593.1532 | 5.40 | 3.383 | 1, 3 | 1 |
| 51. | hispidulin 7-O-rutinoside | C28H32O15 | 607.1675 | 6.34 | 1.049 | 1, 2, 3 | 2 |
| 52. | pectolinarin (pectolinarigenin 7-O- rutinoside) a |
C29H34O15 | 621.1824 | 7.67 | −0.199 | 1, 2, 3 | 1 |
| Free fatty acids | |||||||
| 53. | nonanedioic acid (azelaic acid) | C9H16O4 | 187.0967 | 6.32 | −4.502 | 1, 2, 3 | 2 |
| 54. | 3-hydroxysuberic acid | C8H14O5 | 189.0758 | 4.64 | −5.483 | 1, 2, 3 | 2 |
1,3B− (147.045). In addition, the neutral losses of 16 Da (CH4), 14 Da (CH2) and 28 Da (CO) afford fragment ions in the low mass range at m/z 136.988 (1,3A−-CH4-CO) and m/z 117.028 (1,3B−-CH2) (Table 1 and Table S1, Figure 3).
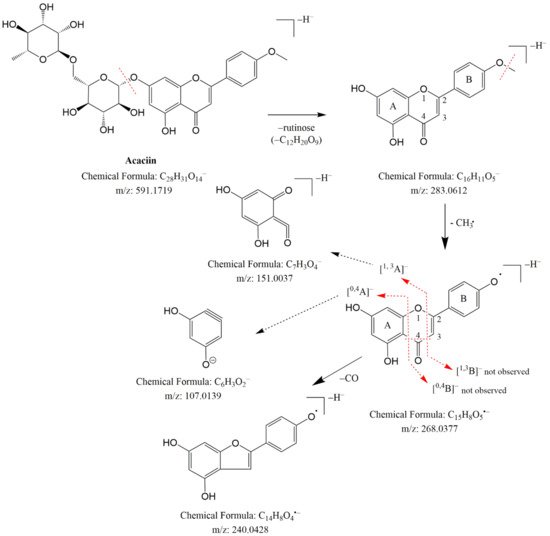

Figure 3. Fragmentation pathway of acaciin (49).
These data corresponded to the Justesen key for methoxylated flavonoid dereplication [23] and the detailed analysis of the fragmentation pathway of methoxylated flavonoids done by Ren et al. [24]. Thus, 34 was identified as pectolinarigenin (Table 1). By analogy with 34, 35 gave fragment ions at m/z 136.986 (1,3A−-CH2-H2O-CO) and 1,3B− at m/z 133.028, indicating a methoxyl group in the A ring. Compound 35 was ascribed to nepetin, previously isolated from Cirsium species [8]. The isobaric pair 28/29 afforded [M-H]− at m/z 283.061 and fragment ions at m/z 268.037 [M-H-•CH3], 240.043 [M-H-•CH3-CO]−and 239.034 [M-H-CO2]−, indicating methoxylated flavonoids. A free hydroxyl group in the B ring of 28 was deduced from the RDA fragment ion at m/z 117.033 (1,3B−), while a diagnostic ion at m/z 165.349 (1,3A−), corresponding to the methoxyl group, was deduced in the A ring. These data are consisted with Justesen [23] and 28/29 were ascribed to genkwanin (4′,5-dihydroxy-7-methoxyflavon) and acacetin (5,7-dihydroxy-4′ methoxyflavon), respectively (Table 1 and Table S1).
Two peaks (31 and 32) produced the same [M-H]− at m/z 299.056 and a fragment ion at m/z 284.032 [M-H-•CH3]− (Table 1 and Table S1). Compound 32 gave diagnostic ions at m/z 256.035 [M-H-•CH3-CO]−, 151.002 (1,3A−) and 107.012 (0,4A−), indicating that the methoxy group is situated in the B ring. Compound 31 yielded a relevant fragment ion at m/z 136.988 (1,3A−-CH4-CO), corresponding to a methoxyl group at C-6 in the A-ring. Thus, 31 and 32 could be related to hispidulin and diosmetin, respectively (Table 1 and Table S1) [23]. In the same manner, 36 afforded diagnostic ions at m/z 314.043 [M-H-•CH3]−, 299.019 [M-H-2•CH3]−, 271.025 [M-H-2•CH3-CO]− and 227.035 [M-H-2•CH3-CO-CO2]− together with RDA fragments at m/z 161.023 [1,3A−-CH4-H2O]− and 151.002 [1,3A−-CH4-CO]. Accordingly, 36 could be associated with cirsiliol, previously determined in Cirsium species [8].
By analogy to flavones and flavonols, the glycosides of methoxylated flavonoids 43–49 and 51–52 were ascribed. MS/MS spectra of 49 and 52 revealed base peaks corresponding to the simultaneous loss of hexose and deoxyhexose. The aglycone of 49 showed a fragmentation pathway similar to acacetin (29) [23], while 52 corresponded to pectolinarigenin (34). Thus, 49 and 52 were identified as rutinosides acaciin and pectolinarin, respectively. The identification of 31, 49 and 52 was confirmed by comparison with reference standards (Table 1 and Table S1).
2.2. Total Content of Phenolics and Flavonoids
Polyphenols and their biological properties are one of the most attractive topics in the natural sciences. Nowadays, humanity needs to substitute synthetic compounds with natural ones. This means safe and alternative raw materials need to be found [25][27]. In this sense, total phenolic and flavonoid content is considered a first insight in evaluating plant extracts. Thus, the total amount of these biocompounds in tested extracts were determined by using spectrophotometric methods (Table 2). Root extract was found to have the highest content of phenolics (143.62 mgGAE/g), followed by flower heads and aerial parts (71.75 ± 1.47 mgGAE/g and 26.02 ± 1.49 mgGAE/g, respectively).
Table 2. Total bioactive compounds and antioxidant properties of Cirsium appendiculatum extracts *.
| Parts | Total Phenolic Content (mgGAE/g) | Total Flavonoid Content (mgRE/g) | DPPH• (mgTE/g) | ABTS•+ (mgTE/g) | CUPRAC (mgTE/g) | FRAP (mg TE/g) |
PHMD (mmolTE/g) | Metal Chelating (mgEDTAE/g) |
|---|---|---|---|---|---|---|---|---|
| Flower heads | 71.75 ± 1.47 b | 46.59 ± 0.89 a | 101.79 ± 0.15 a | 224.57 ± 0.57 a | 356.97 ± 11.52 b | 169.60 ± 0.84 b | 1.71 ± 0.07 b | 32.53 ± 3.51 a |
| Aerial parts | 26.02 ± 1.49 c | 2.64 ± 0.08 c | 70.25 ± 1.91 c | 124.16 ± 4.73 b | 103.77 ± 5.89 c | 69.98 ± 2.01 c | 0.74 ± 0.01 c | 9.42 ± 0.54 b |
| Roots | 143.62 ± 2.99 a | 3.99 ± 0.06 b | 97.95 ± 0.60 b | 224.59 ± 0.33 a | 618.36 ± 5.17 a | 269.89 ± 8.50 a | 3.36 ± 0.15 a | na |
| 2 | ||||||||
| 44. | ||||||||
| hispidulin 7- | ||||||||
| O | ||||||||
| -hexoside | ||||||||
| C | ||||||||
| 22 | ||||||||
| H | ||||||||
| 55. | ||||||||
| 3-hydroxyazelaic acid | ||||||||
| C | ||||||||
| 9 | ||||||||
| H | ||||||||
| 16 | ||||||||
| O | ||||||||
| 5 | ||||||||
| 203.0918 | ||||||||
| 6.25 | ||||||||
| −3.677 | ||||||||
| 1, 2, 3 | ||||||||
| 2 | ||||||||
| 56. | ||||||||
| 2-dodecenoic acid | ||||||||
| C | ||||||||
| 12 | ||||||||
| H | ||||||||
| 20 | ||||||||
| O | ||||||||
| 4 | ||||||||
| 227.1287 | ||||||||
| 9.46 | ||||||||
| −0.715 | ||||||||
| 2, 3 | ||||||||
| 2 | ||||||||
| 57. | 9,13-dyhidroxyoctadeca-9,11,13-trienoic acid | C18H30O4 | 309.2074 | 12.76 | 0.768 | 2, 3 | 2 | |
| 58. | 11,12-dyhidroxyoctadeca-9,13,15-trienoic acid | C18H30O4 | 309.2075 | 12.91 | −0.332 | 2 | 2 | |
| 59. | 9,10-dyhidroxyoctadeca-12,14,16-trienoic acid | C18H30O4 | 309.2074 | 10.81 | 1.835 | 2 | 2 | |
| 60. | 9,13-dyhidroxyoctadeca-11,13-dienoic acid | C18H32O4 | 311.2231 | 13.67 | 0.859 | 1, 2, 3 | 2 | |
| 61. | 9,10-dyhidroxyoctadeca-9-enoic acid | C18H34O4 | 313.2388 | 13.79 | 0.885 | 3 | 2 |
a Compare to reference standards. 1–flower heads; 2–aerial parts; 3–roots.
In the biochemometric approach, peak areas (log (peak area)) for a data quantitative analysis were used. A designed graph clearly shows the differences of the phytochemical components’ distribution in the studied herbal extracts (Figure S1). Thus, flower heads contained more flavonoids compared to aerial parts and roots. Subsequently, a qualitative analysis was carried out.
2.1.1. Carboxylic (Including Hydroxybenzoic, Hydroxycinnamic and Acylquinic) Acids and Their Glycosides
Hydroxybenzoic (1–4) and hydroxycinnamic acids (5 and 8), their glycosides (9–15) and quinic acid (6) were identified based on a comparison with reference standards and literature data (Table 1 and Table S1). The tentative structure of compound 8 was deduced from fragment observation at m/z 197.045 [syringic acid-H]− and 123.007 [syringic acid-H-CO2-2CH3]−, referring to the elimination of caffeoyl moiety (−162.05 Da) and eased its preliminary assignment as caffeoyl-syringic acid [2]. The MS/MS spectrum of 10 revealed a neutral loss of deoxyhexose (−146.09 Da), and diagnostic fragment ions for vanillic acid at m/z 167.034 [vanillic acid-H]−, 152.010 [vanillic acid-H-CH3]− and 123.043 [vanillic acid-H-CO2]−, and were tentatively ascribed to vanillic acid O-deoxyhexoside (Table 1 and Table S1) [14]. In the same manner compounds 7 [15], 8 [16], 11 [17], 12 [18], 14 [19] and 15 [20] were tentatively annotated (Table 1 and Table S1). The isolation of 8 was not reported in the literature and therefore it could be referred to as “unknown”.
The acylquinic acid dereplication was based on conformity with the structure-diagnostic hierarchical keys for chlorogenic acid identification proposed by Clifford et al. [21] and later developed by Jaiswal et al. [22], as well as literature data acquired by hybrid Q-Orbitrap mass spectrometry [2]. Thus, six mono-acylquinic (16–21), four di-acylquinic (22–25) and one tri-acylquinic (26) acids was annotated in the studied extracts.
2.1.2. Flavonoids, Flavones and Flavonols
The aglycones apigenin (27), luteolin (30) and quercetin (33) were deduced from the Retro-Diels-Alder (RDA) cleavages 1,3A− at m/z 151.002, 1,3B− at m/z 117.033 (27) and 133.028 (30), 0,4A− at m/z 107.012, 1,2A− at m/z 178.998 (33) and 1,2B− at m/z 121.028 (33) (Table 1 and Table S1) [2]. The fragmentation pathways of 38, 40 and 50 showed neutral mass losses of deoxyhexose (146.059 Da), hexose (162.053 Da) and rutinose (308.112 Da). Consequently, the aglycone was registered at m/z 285.041 together with the radical aglycone at m/z 284.033 [Y-H-]−•, as usually seen in flavonoids substituted at the 3-position, i.e., flavonol 3-O-glycosides. Fragment ions at m/z 255.030 and 227.035 resulting from neutral losses of CH2O and CO, respectively, being present in high abundance corroborated kaempferol [2]. MS/MS spectra of 39 and 42 demonstrated neutral mass losses of hexuronic acid (176.033 Da) together with base peaks at m/z 269.045 and 285.0403. Accordingly, 39 and 42 could be associated with apigenin O-hexuronide and luteolin O-hexuronide. Compounds 27, 30, 37, 40 and 41 were unambiguously identified by a comparison with retention times in LC-MS and fragmentation fingerprints of the reference standards.
2.1.3. Methoxylated Flavonoids
MS/MS spectrum of 34 revealed fragment ions at m/z 298.048 [M-H-•CH3]− and 283.025 [M-H-2•CH3]−, indicating a subsequent loss of two methyl radicals. A series of fragment ions resulting from neutral losses were registered at m/z 255.029 [M-H-2•CH3-CO]−, 227.034 [M-H-2•CH3-2CO]−, 211.039 [M-H-2•CH3-CO-CO2]− and 183.044 [M-H-2•CH3-2CO-CO2]− (Table 1 and Table S1). A methoxy group at C-6 (ring A) and another one in ring B were deduced from the lack of the initial RDA ions 1,3A− (181.014) and
* Values are expressed as mean ± S.D (n: 3). GAE: Gallic acid equivalent; RE: Rutin equivalent; TE: Trolox equivalent; EDTAE: EDTA equivalent; na: not active. Different letters (a, b and c) indicate significant differences in the extracts (p < 0.05)
Regarding total flavonoids, the values are in the following order: flower heads (46.59 mg RE/g) > roots (3.99 mg RE/g) > aerial parts (2.64 mg RE/g). Hence, flavonoids represent about 50% of the total phenolic components in flower head extract. According to a literature survey, different levels of total bioactive compounds in Cirsium species were observed [26][27][28,29]. These differences could be linked to the habitat of the studied plant, climate conditions or extraction procedures/solvents. However, in past years, the utilization of spectrophotometric methods for total content of bioactive compounds has led to some concerns, and these methods are not used by most scientists anymore [28][30]. Thus, plant matrices are very complexed and phenolics as well as other components such as peptides could be reacting with Folin’s reagent. Finally, the exact quantity of bioactive constituents has to be confirmed by chromatographic techniques such as LC–MS/MS, NMR and Q–TOF-MS analysis.
2.3. Antioxidant Properties
In the present study, C. appendiculatum extracts were tested for antioxidant potential (Table 2). DPPH• and ABTS•+ were used to evaluate radical scavenging ability. The root (97.95 mg TE/g for DPPH• and 224.59 mg TE/g for ABTS•+) and flower head extracts (101.79 mg TE/g for DPPH• and 224.57 mg TE/g for ABTS•+) displayed the strongest abilities. The aerial parts extract had the lowest capacity in both scavenging assays. The reduction abilities of the studied herbal extracts were evaluated using the CUPRAC and FRAP methods, and they are closely linked to the electrohern-donating potential of the extracts. The most prominent reduction ability was observed in the roots, followed by the flower heads and aerial parts. In terms of reduction of Mo (VI) in the phosphomolybdenum (PHMD) assay, the extracts can be ranked as follow: roots > flower heads > aerial parts. In general, the antioxidant data showed the same trends in total phenolic levels. This fact was supported by several authors who reported a strong correlation between total phenolics and radical scavenging and reducing abilities [29][31]. However, the metal chelating method based on the binding of transition metals by phytochemicals did not correlate with the other antioxidant methods. Regarding the metal chelating assay, the best ability was registered in the flower head extract (32.53 mg EDTAE/g), while the root sample was not active.
2.4. Enzyme Inhibitory Effects
Nowadays people are battling noncommunicable illnesses like diabetes mellitus, obesity and Alzheimer’s. In particular, changes in lifestyle and dietary preferences increase the risk of these diseases. In the course of scientific study, some enzymes can be valuable tools against these health problems [30][32]. This approach is known as the enzyme inhibitory theory, in which some enzymes play a role in the pathologies of these diseases. For example, amylase and glucosidase are the main targets for controlling blood sugar levels in diabetes patients [31][33]. In addition, lipase is the main target for controlling obesity. In the present study, the inhibitory effects of different enzyme classes were investigated. Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) belong to the same structural class of proteins, the esterase/lipase family, amylase and glucosidase are hydrolases, while tyrosinase is an oxidoreductase enzyme. Given this information, several compounds have been chemically produced as inhibitors. Although they are accepted as effective agents in the control of global health problems, concerns have been raised regarding some disturbances to wellbeing [32][34]. In this regard, plants are considered to be the most important and richest natural source of enzyme inhibitors such as alkaloids, phenolic acids and terpenoids. Recent studies have shown that some plants and their constituents showed promising inhibitory effects on key enzymes that have been linked to significant health problems [33][35]. Hence, the enzyme inhibitory properties of C. appendiculatum extracts were examined (Table 3). In both AChE and BChE inhibition assays, the root extract exhibited the highest inhibitory values (4.93 mg GALAE/g and 3.80 mg GALAE/g, respectively). The lowest abilities were recorded for aerial parts and flower head samples. Regarding tyrosinase inhibition ability, all extracts showed inhibitory effects, and the values ranged from 97.78 to 127.99 mg KAE/g in the following order: flower heads < aerial parts < roots. All the tested herbal extracts had similar amylase inhibition capacity (p > 0.05), while the strongest glucosidase ability was observed in root extract (0.72 ± 0.07 mmolACAE/g). In addition, the aerial parts sample was not active in glucosidase.
Table 3. Enzyme inhibitory properties of Cirsium appendiculatum extracts *.
| Parts | AChE Inhibition (mgGALAE/g) | BChE Inhibition (mgGALAE/g) | Tyrosinase (mgKAE/g) | Amylase (mmolACAE/g) | Glucosidase (mmolACAE/g) |
|---|---|---|---|---|---|
| Flower heads | 4.40 ± 0.40 a | 1.54 ± 0.07 c | 97.78 ± 0.76 c | 0.60 ± 0.01 a | 0.41 ± 0.10 b |
| Aerial parts | 3.52 ± 0.31 b | 2.67 ± 0.34 b | 110.61 ± 0.79 b | 0.61 ± 0.06 a | na |
| Roots | 4.93 ± 0.25 a | 3.80 ± 0.26 a | 127.99 ± 0.68 a | 0.62 ± 0.04 a | 0.72 ± 0.07 a |
* Values are expressed as mean ± S.D (n: 3). GALAE: Galatamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent; na: not active. Different letters (a, b and c) indicate significant differences in the extracts (p < 0.05)
The data could be related to the different chemical components which were identified in the tested extracts (Table 1). For example, flavonoids and acylquinic acids dominate in the chemical profiles. Several flavonoids, including quercetin, luteolin and apigenin have been described as significant enzyme inhibitors [33][35]. In addition, chlorogenic acid and its derivatives are known to be important neuroprotectors and antidiabetic agents. Some data on the enzyme inhibitory properties of Cirsium species have been found [27][29]. Thus, the obtained data in the current study can be a valuable contribution to the development of new active substances agents against Alzheimer’s disease, and diabetes and its complications. The methoxylated flavone derivatives pectolinarin and its aglycon pectolinarigenin are important for the pharmacological activity of the genus [8]. The aforementioned are responsible for the control of diabetes and other metabolic disorders, and are prominently represented in the chemical composition of C. japonicum [34][36]. Pectolinarin and its aglycone individually significantly reduce glucose levels, but the strongest antidiabetic effect is achieved when they are combined [34][36].
