Arsenic contamination of the environment is a serious problem threatening the health of millions of people exposed to arsenic (As) via drinking water and crops grown in contaminated areas. The remediation of As-contaminated soil and water bodies needs to be sustainable, low-cost and feasible to apply in the most affected low-to-middle income countries, like India and Bangladesh. Phytoremediation is an aesthetically appreciable and successful approach that can be used for As decontamination with use of the best approach(es) and the most promising plant(s). However, phytoremediation lacks the required speed and sometimes the stress caused by As could diminish plants’ potential for remediation. To tackle these demerits, we need augment plants’ potential with appropriate technological methods including microbial and nanoparticles applications and genetic modification of plants to alleviate the As stress and enhance As accumulation in phytoremediator plants.
1. Introduction
Arsenic (As) contamination of the soil and water is a serious problem in several parts of the world, especially in South and Southeast Asian countries. It is an issue of concern owing to the toxic impacts of As on plants and humans and due to the span of the affected areas being very large
[1]. The contamination of As has been caused mainly by biogeochemical processes in countries in South and Southeast Asia and by industrial and agricultural processes in European and North American countries
[2][3][4][2,3,4]. The severely affected countries of South and Southeast Asia are renowned for intensive rice cultivation along with the dense population
[5]. Thus, if even a single well or hand pump is contaminated with As in an area, a large number of people are affected. Further, rice cultivation is performed for two seasons or even three seasons in a year with the use of groundwater plus rainwater for irrigation. Therefore, when the groundwater in the area has As contamination, its use for irrigation adds a huge amount of As to the soil every year
[6][7][6,7]. Another important point to consider is the fact that rice is the best-known accumulator of As among crop plants
[8].
The availability, solubility and toxicity of different forms of As depend on the pH, ionic conditions, phosphorous and other elemental contents in the environment, whereas differences in uptake rates contribute to the degree of cellular exposure to arsenic. A majority of As released into the environment is inorganic and is accumulated by binding to organic soil matter. In an aerobic environment, mostly the arsenate [As(V)] form predominates, whereas the arsenite [As(III)] form is predominant in anaerobic conditions. A higher As(III) contamination in paddy fields due to water logged conditions and the presence of a potential As(III) accumulator plant, rice, are both of serious concern
[9][10][9,10].
The problem of As contamination is the need for use of sustainable and low-cost solutions for the remediation of groundwater and soil
[5][11][5,11]. There are several physical and chemical methods for the treatment of contaminated water and soil
[12]. The natural microbial or plant-based approaches are known as bioremediation and phytoremediation, respectively. These methods are dependent on natural resources (minerals, water and solar energy) and therefore cost less and do not add any xenobiotics
[13]. However, both methods have merits and limitations. The treatment of huge amounts of water/soil under in situ conditions by physico-chemical methods would be extremely costly
[14], while the use of plants for this purpose would make the process very slow. In this regard, any method should have feasibility for application at the site itself, low-cost and be sustainable. Therefore, future research endeavors will require an optimum integration of physico-chemical and biological methods for effective sustainable remediation of contaminated areas.
Plants enhance soil fertility and enrich microorganisms of the soil during the course of remediation. In addition, the application of economically useful plants in phytoremediation makes it feasible for farmers to adopt it
[15]. Plants with a faster growth rate, high biomass, and high shoot As accumulation are desirable for phytoremediation
[16]. However, it has been difficult to find all three qualities in one plant. The plants with high As accumulation in shoots and a short life cycle have been found to have low biomass, while there are other plants which have high biomass but accumulate As with low efficiency
[17]. Further, some high biomass economically useful plants suffer from As toxicity and cannot grow at their full potential. To overcome such difficulties, microbial association as a sustainable strategy has been utilized to enhance the growth and biomass of plants and to enhance their As accumulation efficiency
[18][19][18,19]. Currently, the application of nanoparticles has become an acceptable approach for the reclamation of polluted ecosystems
[20][21][22][20,21,22]. The concept of nano-phytoremediation technology has been emerging for the removal of contaminants from soil/water, which involves the application of both nanotechnology and phytoremediation
[23][24][25][26][23,24,25,26]. However, the main challenge in using nanoparticles for the remediation of pollutants is the lack of an adequate number of reports proving its efficacy.
2. Phytoremediation: A Sustainable Approach
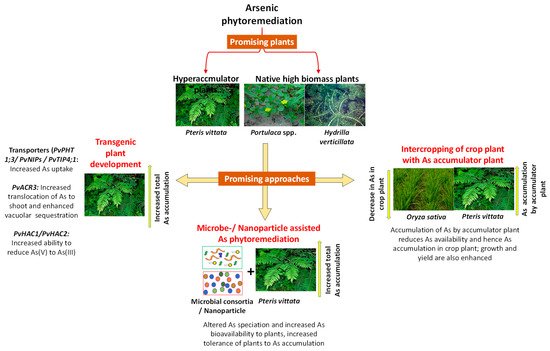
There are various approaches of As phytoremediation that can be utilized judiciously for remediation of contaminated sites. Various approaches are summarized in Figure 1 and are discussed below. Recent studies demonstrating the potential of various approaches have been presented in Table 1.
Figure 1. Various approaches to arsenic phytoremediation: use of hyperaccumulator plants or native high biomass and bioenergy plants; intercropping of arsenic accumulator plant with a crop plant for reduced arsenic toxicity to crop plant; microbe-or nanoparticle-assisted arsenic phytoremediation and the use of genetic engineering approaches to enhance phytoremediation potential of plants.
Table 1. A summary of recent studies on various phytoremediation approaches.
| Plants |
Arsenic Stress |
Results |
Ref. |
| Arsenic Hyperaccumulator Plants |
| Landolita punctata |
As(V) (0.5–3.0 mg/L | | ) |
Plants showed As hyperaccumulation (>1000 mg/kg As) at or more than 1 mg/L As; however, higher than 1 mg/L As levels were toxic |
[27] |
| Pteris vittata |
As (average 8885 mg/kg) and thallium (3.91 to 178 mg/kg) contaminated mining area |
Pteris vittata | accumulated around 7215–11,110 mg/kg As, and 6.47–111 mg/kg of thallium |
[28] |
| High Biomass Producing Plants |
| Calatropis prosera |
Arsenic given in hydroponic and soil |
C. procera | reduced As concentration by 45% and 58% in hydroponics and by 30% and 36% in soil, after 15 and 30 days, respectively. |
[29] |
| Portulaca oleracea |
As (154 mg/kg and 193 mg/kg at site-I and site-II); other metals (Cd, Pb, Cu) were also present |
At site I, As accumulation in stem was around 94.5 mg/kg, whereas at site II, it was 73.6 mg/kg |
[30] |
| Plants with Economic Utiliity |
| Helianthus annus |
Farmland soil containing As (84.85 mg/kg) |
The mean As level 49.04 mg/kg in the above-ground parts. Average seed yield (45.90 kg/m | 2 | ) and oil production (34.65%) |
[31] |
| Hydrilla verticillata |
As(V) (15–375 μg/L) |
Total As accumulation was 197.2 μg/g dry weight when As(V) was 375 μg/L |
[32] |
| Microbe-Assisted Arsenic Remediation |
| Arundo donax | + consortia of two strains of | Stenotrophomonas maltophilia | and one strains of | Agrobacterium | sp. |
As(III) (2–20 mg/L) |
In the presence of bacterial consortium, 11.37 mg/kg As was volatilized by transpiration |
[33] |
| Alfalfa | + | Ensifer | sp. M14 |
Soil As(III) (10 mg/kg) |
As concentration in leaves of inoculated plants was 11% higher than those cultivated without microorganisms. |
[34] |
| Nano-Phytoremediation Approaches |
| Eucalyptus leaf extract mediated synthesis iron oxide NPs |
Arsenic |
Arsenic adsorption capacity was found to be 39.84 mg/g |
[35] |
| Isatis cappadocica + | glutathione modified superparamagnetic iron oxide NPs {nFe | 3 | O | 4 | @GSH} |
Soil As (1000 μM) |
nFe | 3 | O | 4 | @GSH treatment increased growth of plants and As tolerance by reducing As accumulation in plants |
[36] |
| Genetic Engineering Approaches |
| Arabidopsis thaliana | transformed with bacterial As transporter (ArsB) targeted to vacuolar membrane |
As(III) (5 μM) |
Transgenic plants showed higher As accumulation in shoots compared to wild type plants |
[37] |
| Nicotiana tabaccum | transformed with | PvPht1;3 | from | P. vittata |
As(V) (20 μM)
Soil As (9.66 mg/kg) |
Arsenic accumulation in shoot tissues of transgenic tobacco increased in both hydroponic and soil experiments |
[38] |
2.1. Selection of Plants for Arsenic Phytoremediation
2.1.1. Arsenic Hyperaccumulators
Hyperaccumulator plants can accumulate metal in their shoots beyond a certain threshold limit, which is 1000 mg/kg for As
[39]. Further, the bioaccumulation factor (BF; indicative of soil to plant metal transfer) and translocation factor (TF; indicative of root to shoot metal transfer) are also considered while categorizing a plant as a hyperaccumulator
[40]. Both BF and TF should be more than one (>1) for an As hyperaccumulator plant. Hyperaccumulation of As has been observed mostly in fern plants of the
Pteris genus like
P. vittata [40],
P. longifolia [41],
P. quadriaurita,
P. cretica,
P. ryiunkensis [42], etc. and
Pityrogramma calomelanos [43]. One of the plants from the Brassicaceae family,
Isatis cappadocica, shows As hyperaccumulation
[44].
P. vittata has worldwide distribution from North America to Europe and Asia and can grow in a wide range of environmental conditions ranging from temperate to tropical
[45].
Arsenic can make up to about 2% of the biomass of
P. vittata [40].
P. vittata is a perennial plant and, therefore, plantation of a field does not need replantation, and harvesting and collection of fronds is needed at regular intervals. Several studies have focused on the use of
P. vittata for the remediation of As-contaminated soil in laboratory, pot and field studies
[46]. Liao et al.
[47] found that from soil containing 64 mg/kg As,
P. vittata removed 7.8% of the As in seven months.
P. vittata plants showed higher As accumulation when grown in soil with added phosphate rock than in soil without phosphate rock amendment
[48]. Phosphorus addition in the form of phosphate rock induces mobilization of As to some extent that, in turn, helps to induce As removal by
Pteris plants
[49][50][49,50].
In a pilot-scale study
[51],
P. vittata was used to minimize As concentration from drinking water through a continuous phytofiltration system. During the 3 month experimental period, up to 1900 L/day water with an initial As concentration of 10.2 μg/L was remediated and was found to contain As concentrations as low as 2 μg/L. The fronds of
P. vittata accumulated 66–407 mg/kg As
[51]. Groundwater remediation has also been demonstrated with the use of
P. vittata [52]. The authors tested the efficiency of one to four
Pteris plants per container of 30 L and with variable nitrogen and phosphorus supply to remediate groundwater containing 130 μg/L As. The As concentration was reduced to less than 10 μg/L in 3 weeks with 4 plants while in 4–6 weeks with 1–2 plants. When fully grown plants with a high root density were reused, one plant per container gave good results. In a recent study,
P. vittata was used in a hydroponic system without any mechanical aeration. The method used was simple in that the plants were grown with rhizomes over the water surface and nutrients were given in a low amount for achieving root proliferation (500 mm root length in four months). From a variable initial water As concentration of 50 μg/L, 500 μg/L, and 1000 μg/L,
Pteris plants could bring down the concentration to 10 to 0.1 μg/L in 1–5 days, 4–6 days and 8–10 days, respectively
[53]. The results suggest the potential of
P. vittata for phytoremediation purposes; however, the use of
P. vittata has been mostly in hydroponics limited to pilot-scale studies. Extension of the approach to field conditions will necessarily require higher biomass development of large scale hydroponic systems, large amounts of water for treatment, and maintenance with optimum nutrient supply and regular cleaning.
2.1.2. High Biomass Plants for Arsenic Cleanup
The remediation of a site in a short time warrants the need of high biomass plants with moderate to high As accumulation and a short life cycle enabling harvesting followed by the use of the field for subsequent cropping of the same or other appropriate plants. This would enable cultivation of phytoremediator crops in a contaminated field throughout the year in changing weather conditions. Some of the high biomass plants with good potential for As accumulation include
Jatropha curcas [54], shrub willow (
Salix spp.), sunflower (
Helianthus annuus)
[55] and Indian mustard (
Brassica juncea)
[56]. In a small field study, sunflower plants were exposed to different As levels in three soil types (sandy, loamy, and clayey) and As accumulation was found to vary from 270 mg/kg to 408 mg/kg in roots, 13 mg/kg to 28 mg/kg in stem and 35 mg/kg to 68 mg/kg in leaves in different soil
[57]. The application of
Salix in phytoremediation has been demonstrated
[58]. Invasive plants like
Parthenium hysterophorus can also be successfully used in remediation strategies as they can grow and cover an area at rapid rates in a wide range of environments and accumulate metals in high amounts
[59]. Favas et al.
[60] found
Callitriche lusitanica to be a potential As accumulator with As concentrations reaching up to 2346 mg/kg DW. Other potential accumulators in higher plants have been identified in lab and field studies, e.g.,
Isatis cappadocica [44],
Sesuvium portulacastrum [61], and
Eclipta alba [62].
Sesuvium is a halophytic plant with a high tolerance not only to salt but also to a number of metals and showed As accumulation 155 µg/g dw upon exposure to 1000 µM As(V) in 30 d
[61].
The contaminated water bodies may be remediated with the help of high biomass aquatic plants like
Ceratophyllum demersum [63],
Hydrilla verticillata [64],
Lemna gibba [65],
Lemna minor [66],
Azolla caroliniana [67],
Pistia stratiotes [68],
Salvinia natans [69] and
Eichhornia crassipes [70].
Lemna gibba has been demonstrated to accumulate As up to 1022 mg/kg dry biomass in 21 d from contaminated surface water containing 41.37–47 µg/L As. The biomass accumulation and As removal potential of
L. gibba were found to be as high as 73.6 t/ha/y and 752 kg As/ha/y, respectively
[65]. In another study,
E. crassipes was found to accumulate about 498 mg As/kg dry weight from a solution of 0.5 mg/L As in 10 d with a reduction of initial As concentration by 83%
[71].
H. verticillata plants were found to remove up to 72% of As from 8 L As (1500 ug/L) medium in 45 d with the maximum As concentration of 388 μg/g dry weight
[72]. These plants show fast growth and high biomass accumulation, can be easily harvested and can reestablish themselves. Aquatic plants also need very little input for growth and have high tolerance to waste water. The use of water fern,
Mircanthemum umbrosum, in As and Cd remediation was studied by Islam et al.
[73]. The use of emergent aquatic plants like
Cyperus vaginatus and
Vetiveria zizanioides has also been demonstrated in phytoremediation studies
[74]. With the use of a high biomass moderate As accumulator, the effective removal of As per year can be higher than that achieved with a low biomass hyperaccumulator. For example, the calculation of yearly As removal by
Sesuvium was found to be as high as 1955 g As/ha/yr at 500 µM As, which was higher than the calculated As removal by
Pteris (525–1470 g As/ha/yr)
[61].
2.1.3. Plants with Bioenergy Potential and Economic Utility
Besides plant biomass, the economic value of the plant system such as high value metabolites, biofuel generation, compost formation, etc. is now considered as one of the prime criteria for selecting plants for phytoremediation. With such an approach, farmers can move from normal cropping patterns to phytoremediator plants
[17][75][17,75]. Plant-based waste material can also be successfully reutilized in remediation projects. This approach not only handles the problem of plant waste utilization at one end but also remediates the contaminated site on the other. Rice husk, mustard husk, coconut coir waste, crop straw, etc. are some of the examples of materials derived from plant materials that can act as biosorbents and remediators of As and can sustain soil fertility and reduce As accumulation in crop plants
[76]. The potential of aquatic plants can also be used with judicious controlled and proper management of generated biomass with biodiesel, biogas, biochar, or compost preparation
[77][78][77,78]. Biochar has emerged as one of the most potential plant based materials that have a number of functional groups (hydroxyl, carboxyl, etc.), making it an excellent binder of metals and therefore its application in soil reduces As stress to crop plants. Further, the use of biochar has also been demonstrated in water filtration
[79]. Zhu et al.
[80] designed a biochar plus periphyton-based system for the removal of As from the wastewater. The first phase of the column contained biochar that removed up to 60% of As(III) from wastewater (containing 2 mg/L As(III); flow rate 1 mL/min) while subsequent a periphyton bioreactor enhanced As removal efficiency up to 90–95%.