N-heterocycles, both saturated and unsaturated, are ubiquitous biologically active molecules that are extremely appealing scaffolds in drug discovery programs. Although classical synthetic methods have been developed to access many relevant N-heterocyclic scaffolds, representing well-established and reliable routes, some do not meet the needs of sustainability. In this context, several advances have been made towards the sustainable synthesis of N-heterocycles.
- green catalysis
- -heterocycles
- synthesis
- bioactive compounds
- metal-catalysis
1. Introduction
Both saturated and unsaturated N -heterocycles are prevalent in biologically active molecules and constitute increasingly attractive scaffolds for the development of new medicines ( Figure 1 ). Several indole and azaindole derivatives have been reported as potent cancer agents. Pemigatinib is a representative medicine approved by the FDA [1][2]. Luotonin A and tryptanthrin are bioactive quinazolines and quinazolones-based alkaloids. Quinazoline derivatives exhibit several activities such as antibacterial, antitubercular and antiviral and are potential inhibitors of epidermal growth factor (EGF) and tyrosine kinase receptors [2][3][4][5][6][7][8][3,4,5,6,7,8,9]. The pyrrole-based statin lipitor is considered as a ‘blockbuster’ drug being widely prescribed, improving the health of millions [9][10] and acting as a cholesterol-lowering drug.

Additionally, saturated N -heterocycles such as ritalin and veliparib are medicinally relevant molecules used for the treatment of ADHD (Attention deficit hyperactivity disorder) and as an anticancer, respectively [10][11]. Piperidines are the most important saturated N -heterocycles in therapeutic compounds, followed by piperazines and pyrrolidines [11][1].
Given the widespread interest in N -heterocycles, the synthesis of these compounds has always been among the most important research areas in synthetic chemistry. Classic named reactions have been developed to address the synthesis of N -heterocycles [12]. Despite the utility of the classical synthetic methods, modern developments in synthetic chemistry underline new sustainable synthetic routes focused on environmentally acceptable alternatives to the classic methods [13]. Indeed, a green and simple access to a wide variety of N -heterocyclic compounds is of crucial importance for drug discovery programs. Due to their widespread application and medicinal relevance to N -heterocycles, being present in both natural and synthetic compounds, their synthesis is an important area of research in synthetic chemistry.
This entry covers the most recent contributions to the sustainable synthesis of N -heterocyclic compounds, particularly the green catalytic methods reported in the last five years. Several catalytic methods are presented, involving both homogeneous and heterogeneous catalysts and recyclable catalysts combined with non-traditional activation methods such as microwave (MW) irradiation. Furthermore, examples of the use of green reagents and atom-efficient methods such as one-pot reactions and acceptorless coupling reactions, among others, as the main tools in green synthesis and catalysis are also covered. Herein, the synthesis of N -heterocycles of medicinal relevance using improved and green approaches are reviewed ( Figure 2 ).
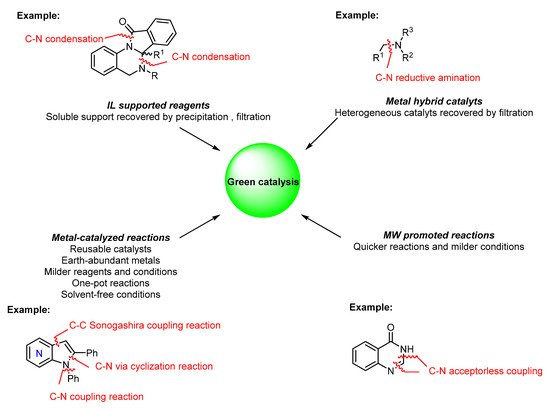
2. Green Catalytic Synthesis of N-Heterocycles
The model reaction involved the coupling of o -aminobenzamide with methanol as both reagent and solvent in the presence of 1 mol% of iridium catalysts and Cs 2CO 3 (0.3 eq.). The reaction was carried out at 150 °C for 12 h and the corresponding product was attained in 13% yield utilizing the [Cp*IrCl 2] 2 (Cp* = pentamethylcyclopentadienyl) catalyst 1 . During the reaction’s optimization, one catalyst was found to have a superior efficacy, resulting in higher overall yields: the [Cp*Ir(2,2′-bpyO)(H 2O)] catalyst 2 ( Scheme 2 ). The authors also found that an increase of Cs 2CO 3 had no effect on the overall yield, so they decided to promote the reaction with MW radiation and ended up with a yield of 88% at 130 °C, under MW for only 2 h.
The cascade procedure involves a palladium-catalyzed N -arylation followed by Sonogashira reaction and subsequent cyclization in a one-pot approach. In order to study the reaction, scope several iodides were employed in the N -arylation reaction and several alkynes were tested in the Sonogashira reactions. The results obtained demonstrate that this methodology exhibits a wide scope and compatibility with electron-withdrawing and electron-donating groups ( Scheme 15 ).
Metal-Organic Frameworks (MOFs) are defined as a class of coordination polymers with permanent porosity [14][54]. These structures have been subjected to particular interest as heterogeneous catalysts, since, in most cases, they allow for the use of milder reaction conditions and a straightforward isolation of the reaction product from the reaction mixture [15][55].
Classical indolization methods were applied for azaindole synthesis; however, the nature of the aminopyridines hampers the efficiency of these methods when compared with anilines [16][45]. Classic synthetic methods remain as an effective way to attain this type of compound; however, the preparation of these scaffolds using this type of reaction is still challenging in terms of sustainability ( Table 1 ). For example, the Bartoli reaction uses nitrobenzenes and vinyl Grignard reagents, and when applied to azaindole synthesis, this method requires a large excess of vinyl Grignard and only affords 4- azaindole and 6-azaindole in generally low yields [17][57]. Furthermore, reactions such as the Hemetsberger-knittel synthesis are limited to few isomers—6-azaindole and 7-azaindole, respectively—and, additionally, can only afford azaindole structures in low to moderate yields [18][19][58,59].
| Azaindole Synthesis | ||
|---|---|---|
| Classical Methods [17][18][19] | Metal-Catalyzed Methods [20][21][22][23] | |
| Advantages | Effective in attaining the product Moderated yields |
One-pot reactions Less waste Compatible with water Mild conditions Atom economy |
| Disadvantages | Limited scope Stoichiometric reagents Strong acids High temperature Poor yields of some isomers |
Catalysts may be expensive Moderate to high yields |
| Scope | Limited to few regioisomers | Access to all regioisomers |
| Energy efficiency | High energy consumption from traditional heating methods | Compatible with MW heating |
| Solvent | Hazardous and chlorinated | Compatible with water |
| N° of steps | At least 2 steps | Maximum 2 steps |
3. Conclusions
This entry collects some of the most recent advances in N -heterocycle synthesis applying more sustainable synthetic procedures. As the pharmaceutical industry is among the most environmentally problematic branches of the chemical industry, the development of green synthetic methods that facilitate the synthesis of heterocycles is mandatory. The recent examples shown include the use of starting materials attached to a soluble support, which allow for a simpler purification process as well as the use of metal hybrid catalysts like MOF that can be separated from the reaction mixture by simple filtration. Furthermore, the use of metal-catalyzed reaction has completely revolutionized the sustainable synthesis of N-heterocyclic structures. The development of novel catalysts based on Earth abundant metals and new reagents has allowed for milder reaction conditions and resulted in the reduced production of hazardous side products. All of these methods represent a step forward in the preparation of important—and often not so easy to prepare— N -heterocycles. Still, some challenges remain regarding the use of more efficient and versatile catalysts that can be applied to a larger scope of starting materials and be compatible with greener reaction media. Catalysis will continue to evolve and attract much attention in sustainable organic synthesis applications.
