1. Introduction
Present in all cells, biomolecular condensates are membraneless organelles (MLOs) containing proteins, ribonucleic acids (RNAs), and other nucleic acids
[1]. These micron-scale macromolecules that can assemble into liquid-like droplets have been proposed to be the origin of life
[2]. Current cell and molecular biology reveal that liquid–liquid phase separation (LLPS) is the driving force behind the assembly or dissolution of biomolecules in energy-efficient, rapid, essential reactions to changing endogenous or exogenous conditions including stress response
[3] and signal transduction
[4,5][4][5], as well as genome expression, organization, and repair
[6]. LLPS creates distinct compartments that enhance or restrict biochemical reactions by enriching or excluding biomolecules from their environment
[7]. Increasing evidence associates diseases such as neurodegeneration and cancer with the formation of protein aggregates from dysregulated, aberrant transitions in phase separation
[8,9,10,11,12][8][9][10][11][12].
Phase separation at its core is a thermodynamic process driven by the reduction or a negative change in global free energy
[1,13][1][13]. LLPS is entropically unfavorable; therefore, multivalent protein–protein interactions that are energetically favorable may be necessary to offset energetic costs
[14]. Adenosine triphosphate (ATP) is the molecule favored by most organisms for capturing and transferring free energy. During hydrolysis, ATP is transformed into adenosine diphosphate (ADP) and inorganic phosphate (Pi). The change in free energy of −7.3 kcal/mol associated with this chemical reaction is used by cells to perform energetically favorable reactions
[15], including relevant post-translational modification (PTM) such as phosphorylation
[16], ubiquitination
[17[17][18],
18], and SUMOylation that may regulate condensate nucleation, composition, and growth
[19,20][19][20]. It is understood that most proteins in the human proteome can undergo LLPS, assembling into dense liquid-like, reversible droplets under most physiological conditions
[21]. Thermodynamic non-equilibrium processes facilitate the constant exchange of substrates and information that allow these condensates to perform important biological functions
[22]. The phase transition of these functionally relevant proteins from their native to droplet states are often mediated and stabilized by ATP-dependent factors such as PTM and RNA. RNAs are critical architectural components that can fine-tune biophysical properties such as viscosity and dynamics in the regulation of spatiotemporal distribution of condensates
[23,24][23][24].
Aberrant phase separation leading to the pathological amyloid fibrillation of fused in sarcoma (FUS), TAR DNA-binding protein 43 (TDP-43), tau, and α-synuclein (α-Syn} are now associated with neurodegenerative disorders such as amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), Alzheimer’s disease (AD), and Parkinson’s disorder (PD)
[26,27,28,29][26][27][28][29]. The timely dissolution of pathological amyloid fibrils may be dependent on cellular levels of ATP, which has recently been identified as a biological hydrotrope
[30]—an amphiphilic molecule that may behave as a surfactant
[31] which can reduce tension between solute and solvent, and increase solubility in an energy-independent manner.
2. ATP Regulates Biomolecular Condensates
At micromolar concentrations in cells, the hydrolysis of ATP phosphoanhydride bonds provides substantial free energy to fuel chemical processes such as post-translational modifications that may maintain fluid phases or facilitate phase separation by generating supersaturation gradients that can induce droplet segregation
[13,15,32,33][13][15][32][33]. At higher physiological concentrations between 2 and 8 mM, ATP becomes a biological hydrotrope that can solubilize proteins to prevent abnormal aggregation and the formation of pathological amyloid fibrils often associated with neurodegenerative disorders such as Alzheimer’s disease (AD)
[30]. Recent extensive all-atom molecular dynamics studies showed that at higher millimolar concentrations (150 mM), ATP prevented the aggregation of amyloid-beta peptide Aβ
16−22 and disrupted prefibril formations
[34], supporting earlier observations of decreased ATP levels in the brain and whole blood of AD transgenic mouse models
[35]. Other experimental studies determined that mechanisms such as the suppressed fibrillation of disordered protein by the adenosine moiety of ATP leading to increased protein stability and reduced thermal aggregation may not be typical of hydrotrope-type reactions. Instead, ATP could be viewed as a kosmotropic anion
[36] that can increase the solubility of the hydrophobic adenine part
[37]; thus, the term “biological aggregation inhibitor” may be more appropriate
[38].
Even though ATP is produced mainly in mitochondria, ATP levels in the mitochondrial matrix are significantly lower than those found in the cytoplasm and nucleus
[39,40][39][40]. Voltage-dependent anion channels (VDACs) located in the mitochondrial outer membrane (MOM)
[41] and adenine nucleotide translocators (ANTs) on the inner mitochondrial membranes (IMM)
[42,43][42][43] facilitate the export of ATP into cytosol where ATP accumulation has been observed to be the highest
[44]. The high physiological concentration of ATP in cytoplasm may be used to control the pathological aggregation of macromolecules that coacervate as a result of transient interactions during LLPS in the cytoplasm and nucleus
[45,46][45][46]. A major hallmark of ALS/FTD is the presence of FUS inclusion in the cytoplasm. FUS are prosurvivor molecules that re-localize from the nucleus to cytoplasm under stress conditions to form reversible, survival-promoting stress granules via LLPS
[47,48][47][48]. Stress granules contain important ATP-dependent RNA helicases that function as ATPases to hydrolyze ATP during assembly and disassembly
[49]. Stress granules could not be formed without the presence of ATP, and the presence of ATP was required to maintain the liquid-like behavior of assembled droplets
[32]. A recent in vitro study showed that aggregate disassembly is also an ATP-dependent process.
During metabolic stress such as nutrient deprivation that causes ATP depletion, cells compartmentalize and sequester misfolded proteins into stress granules to protect cellular fitness. Budding yeast subjected to 0.02% glucose starvation showed a 5-fold ATP decline to ~1.1 mM within 10 min, accompanied by a ~4.4-fold increase in median aggregate diameter, whereas the addition of glucose restored ATP levels, quickly reducing aggregate size and abundance back to control values
[50]. Mutants with abolished ATP hydrolysis failed to dissolve aggregates even when placed back in 2% glucose solutions after starvation
[50]. In the same manner, ATP has been shown to enhance the LLPS of FUS at low concentrations but dissolves FUS aggregates at higher concentrations
[51]. Moreover, 8 mM of ATP complexed with Mg
2+ ions prevented the LLPS of FUS and dissolved previously formed FUS condensates
[30]. The presence of ATP facilitates the essential phase transition of FUS into stress granule droplets, yet prevents further transition into irreversible aggregation and the fibrillation of FUS to cause cytotoxicity by binding to the RNA-recognition motif (RRM) domain of FUS, kinetically inhibiting the fibrillization of FUS
[52]. Similarly, through binding to arginine-containing domains in TDP-43, ATP alters physicochemical properties to induce LLPS, causing droplet formation at molar ratios as low as 1:100 (protein to ATP); by contrast, increasing ATP concentrations could reduce droplet formation, with TDP-43 droplets completely dissolving at a molar ratio of 1:1000
[53]. Nevertheless, in order to completely dissolve the amyloid-beta peptide Aβ-42 associated with AD, supraphysiological concentrations of ATP in excess of 100 mM were found to be necessary
[30].
Tau is the major constituent of fibrillar tangles in AD. Phase-separated tau forms droplets that serve as intermediates toward aggregation
[29]. Physiological concentrations of ATP at 0.1–10 mM enhanced the fibrillation of 10 μM tau K18 (equivalent to 10–1000-fold molar ratio) by accelerating aggregation in a concentration-dependent manner
[54] through energy-independent binding to tau proteins
[55]. It may seem paradoxical that ATP would enhance the formation of amyloids and prions that are associated with diseases. As a matter of fact, prion-like mechanisms are functional biological processes ubiquitously present from bacteria to humans
[56]. The nucleation and growth of amyloid fibrils in FUS, TDP-43, tau and α-synuclein are dependent upon intermolecular interactions of intrinsically disordered regions (IDRs) and proteins (IDPs) such as prion-like domains and low-complexity sequence domains
[57].
Proteins that undergo LLPS often contain long segments that are intrinsically disordered and lack well-defined three-dimensional structure
[58]. The relatively low concentration of hydrophobic amino acids in IDPs enables the rapid exchange between multiple conformations where condensates form without altering the affinity of binding interactions during LLPS
[59,60,61][59][60][61]. Although the formation of biomolecular condensates can potentially accelerate amyloid aggregation, condensates can also inhibit fibril formation by the sequestration of aggregation-prone, prion-like IDPs. Biomolecular condensates derived from proteins associated with the formation of processing bodies (P-bodies) prevented aberrant amyloid aggregation despite local increase in concentration of aggregate-prone proteins
[62]. P-bodies are conserved eukaryotic cytoplasmic ribonucleoprotein (RNP) membraneless organelles that regulate protein homeostasis in non-stressed cells through LLPS involving messenger RNAs (mRNAs) and low-complexity sequence domains
[63,64,65,66][63][64][65][66]. P-bodies respond to cellular stress, especially DNA replication stress, by increasing their sizes and numbers
[67,68][67][68]. The disassembly of P-bodies in yeast is an ATP-dependent process involving ATP hydrolysis by DEAD-box ATPases
[69]. Inhibition of DDX ATPase activity can disrupt the disassembly of physiological MLOs such as P-bodies and stress granules
[69,309][69][309]. To remain in functional states, bimolecular condensates may require energy to support the continuous active restructuring and rearrangement of molecular components. Insufficient or the depletion of ATP can directly impact the physical and functional properties of biomolecular condensates
[32,33,79,118][32][33][79][118].
2.1. Dimerized ATP Synthase/ATPase Require High-Curvature Lipid Domains
First isolated in 1960
[119[119][120],
120], F
1F
0 ATP synthases are mostly localized in the inner membrane invaginations of mitochondria
[121]. Eukaryotes and prokaryotes use four major types of ATPases localized in cell membranes to release energy during hydrolysis of ATP for the maintenance of critical transmembrane ionic electrochemical potential differences
[122]. In the ubiquitous intracellular powerhouses of eukaryotes, F
1F
0 ATP synthase is complex V of the electron transport chain responsible for chemiosmotic oxidative phosphorylation (OXPHOS) that couples ATP synthesis to the inner membrane proton gradient
[123,124,125][123][124][125]. The ATP synthases of mammalian mitochondria are usually arranged in rows of dimeric complexes of two identical monomers located at the highly curved apex of deep IMM invaginations known as cristae
[168]. Dimerized ATP synthases are seven times more active than monomers
[169]. Dimerization of ATP synthase may be a major determinant in cristae formation
[170], because extreme cristae membrane curvature is shaped by the self-assembly of ATP monomers into dimerized rows
[171]. Inability to form dimers resulted in reduced or deformed cristae invaginations
[172] that impacted ATP production from decreased OXPHOS activity as a result of defective cristae morphology
[173,174][173][174]. Experimentally purified ATP synthase reconstituted with membrane lipids revealed that dimerized rows of ATP synthases were formed only on curved surfaces and not on flat membrane areas
[175]. Extracellular F
1F
0 ATP synthases have been observed to translocate from mitochondria to lipid raft domains of various cell types, including plasma membranes of gonadotropes
[176], and the sarcolemma of muscle fibers
[177].
2.2. Translocation of ATP Dimers to Lipid Rafts Are Cellular Responses to Stress and Stimuli
Biomolecular condensates adapt to changing endogenous or exogenous conditions [3] by continuously fine-tuning biochemical reactions, enriching or excluding biomolecules from their environment [7]. The rapid translocation of mitochondrial ATP synthase to lipid rafts may be integral to these adaptive responses because ATP functions not only as a biological hydrotrope [30,178][30][178], increasing the solubility of positively charged, intrinsically disordered proteins [179], but may act as a universal and specific regulator of intrinsically disordered regions (IDRs) capable of altering physicochemical properties, conformation dynamics, assembly, and aggregation [45], in addition to providing phosphates as an energy source to fuel post-translational modifications that regulate the fluctuation of biomolecule phase separation during condensate formation [79,178][79][178]. Failure to maintain nanoscopic lipid raft domains with appropriate line tension and membrane elasticity [185] to functionally host dimerized ATPase [186], ATP synthase [175] may contribute to aberrant phase separation, resulting in pathogenic protein aggregates in neurodegeneration [11] and cancer [10,12][10][12].
The ability of ATP synthase/ATPase to form dimerized rows on the IMM of mitochondria and other membrane surfaces may be highly dependent upon membrane lipid composition [211] and curvature [175]. Uncontrolled, excess oxidative stress can cause lipid peroxidation [212] which induces pathological changes to membrane lipid composition, including alterations of cardiolipin in IMMs [211[211][213],213], as well as changes in membrane curvature that prevent optimal dimerization and the subsequent functioning of ATP synthase/ATPase [214,215][214][215]. Insufficient or depletion of ATP can directly impact the physical and functional properties of biomolecular condensates [32,33,79,118][32][33][79][118]. ATP is not only a biological hydrotrope capable of inhibiting protein LLPS and aggregation at high mM concentrations; it has recently been observed to act as a universal and specific regulator of IDRs, altering their physicochemical properties, conformation dynamics, assembly, and aggregation [45].
3. Melatonin Is a Potent Ancient Antioxidant That Protects ATP Levels to Regulate the Formation and Dissolution of MLOs
Melatonin (
N-acetyl-5-methoxytryptamine) is a mitochondria-targeted molecule found in cells of all tested eukarya and bacteria
[414]. Effective distribution via horizontal gene transfers may explain the discovery of ancient homologs of arylalkylamine
N-acetyltransferase (AANAT), the enzyme responsible for the rhythmic production and release of melatonin in bacteria, fungi, unicellular green algae, and chordates
[415,416,417][415][416][417]. In present-day vertebrates, it is estimated that ~99% of melatonin is likely not produced in the pineal gland, nor released into circulation upon pineal production
[418], but is mainly synthesized and localized in mitochondria
[419,420][419][420]. Photosynthetic cyanobacteria responsible for filling the earth with oxygen that led to the extinction of obligate anaerobes produce melatonin
[421,422][421][422]. The presence of melatonin in primitive unicellular organisms including
Rhodospirillum rubrum and cyanobacteria, precursors to mitochondria and chloroplasts, respectively
[415[415][423][424][425],
423,424,425], may have conferred protection against endogenous and exogenous oxidative stress that could readily damage biomolecules and disrupt ATP production at plasma membranes
[421,425,426,427][421][425][426][427]. This unique feature implies that melatonin may have an intrinsic modulatory effect over phase separation in early organisms.
As in all eukaryotic cells of plants and animals, LLPS is also believed to be the organizing principle behind the subcellular compartmentalization of membraneless organelles (MLOs) in prokaryotic bacteria
[277[277][428],
428], where condensate formation is tightly correlated with ATP levels. Impaired ATP hydrolysis from reduced ATPase activity in bacteria causes droplet formation by phase separation
[429,430][429][430]. Cyanobacteria, the only known prokaryote capable of water oxidation
[431], has recently been shown to exhibit circadian rhythm in the formation and dissolution of MLOs that remain soluble during daylight, but became reversible, insoluble condensates at night. The formation of aggregates allows cyanobacteria to conserve energy when metabolic activities and ATP levels are lowered at night
[432,433,434,435][432][433][434][435]. It is therefore not unexpected that when ATP production was disrupted, insoluble aggregates could be induced to form in cyanobacteria even during daylight by suppressing F
1F
0-ATP synthase or uncoupling OXPHOS with mitochondrial proton gradient inhibitors
[432].
The gene sequences of cyanobacteria ATP synthase subunits are extremely similar to those in chloroplasts
[436]. Embedded in the thylakoid membrane, both ATP synthase in cyanobacteria and chloroplasts (CF
0CF
1) control transmembrane electrochemical proton gradients for the production of ATP
[437,438,439][437][438][439]. Similar to CL, which is synthesized from phosphatidylglycerol (PG) in all organisms
[440], PG is the primary phospholipid associated with photosystem complexes that carry out electron transport reactions during oxygenic photosynthesis
[441]. Both CL and PG are essential for maintaining the proper lipid composition that supports electron transport and ATP production in eukarya and prokarya, although these lipids are easily subjected to damage via lipid peroxidation
[213,234,442,443,444,445,446][213][234][442][443][444][445][446]. The antioxidant effects of melatonin and its metabolites become particularly meaningful when the prevention of CL peroxidation by hydroperoxyl in mitochondrial membranes can affect the formation and dissolution of biomolecular condensates (
Figure 1).
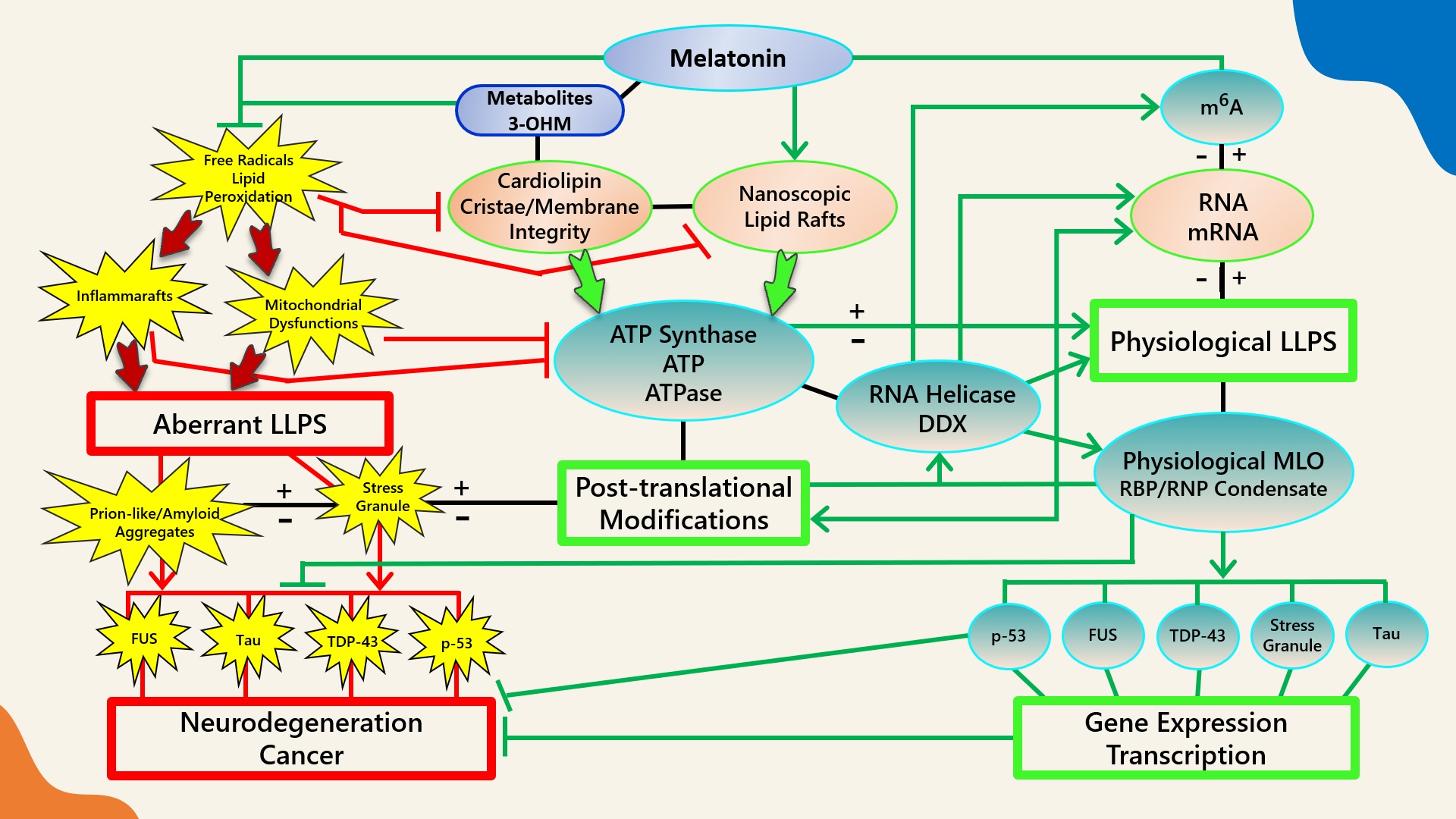 Figure 1.
Figure 1. Schematic illustrating the regulation of biomolecular condensates by melatonin represented through observations reported in antioxidant protection against lipid peroxidation to maintain membrane/lipid raft composition/stability that serves to maintain adequate ATP levels in all cellular compartments to fuel, support, and regulate post-translational/m
6A modifications that may fine-tune RNA dynamics in the assembly and disassembly of MLOs to prevent pathological aggregations in neurodegenerative disorders. LLPS: liquid–liquid phase separation; DDX: Dead-box RNA helicase; m6A: N
6-methyladenosine; MLO: membraneless organelle; RBP: RNA-binding protein; RNP: ribonucleoprotein; PTM: post-translational modification (See Abbreviations for additional acronyms).
3.1. Melatonin Metabolite 3-OHM Inhibits Lipid Peroxidation by Hydroperoxyl Radical
Melatonin and its secondary, tertiary, and quaternary metabolites actively scavenge potent free radicals
[317,426,447][317][426][447] including hydroxyl radicals
[448], singlet oxygen
[449[449][450],
450], hydrogen peroxide
[451], nitric oxide
[452[452][453][454],
453,454], and peroxynitrite anions
[455] via different antioxidant mechanisms such as direct radical trapping in Type I antioxidant reactions and inactivating hydroxyl radicals (
•OH) through the sequestration of metal ions and deactivating
•OH during Fenton-like reactions in Type II antioxidant reactions
[456]. In addition, melatonin and its metabolites collectively preserve the chemical integrity of biomolecules from oxidative stress via Type III antioxidant cellular repair processes and Type IV antioxidant reactions that can enhance antioxidant enzymes and inhibit pro-oxidant enzymes
[456].
A recent study that analyzed the mechanistic interactions between melatonin and
•OH employing density functional theory found that one molecule of melatonin effectively scavenged two
•OH radicals to produce the stable footprint metabolite, cyclic 3-hydroxymelatonin (3-OHM)
[457], in perfect agreement with mechanisms reported in prior experimental and theoretical studies
[448,458,459,460][448][458][459][460]. 3-OHM has been shown to react with hydroperoxyl radicals (
•OOH) at rates 98.4 times faster than Trolox in aqueous solution
[459]. Trolox is a water-soluble, cell-permeable analog of vitamin E with high radical scavenging potential often used as a yardstick for measuring antioxidant capacities in vitro. Trolox resides mainly in the aqueous phase; therefore, it has been observed that Trolox and other water-soluble antioxidants exhibit reduced scavenging activity if radicals are produced within hydrophobic cores of lipid membranes
[461]. Melatonin accumulates in all of the internal membranes of cells as well as other hydrophobic sites
[412]; therefore, this antioxidant may be uniquely positioned for quenching lipid peroxidation by
•OOH and other free radicals that penetrate deep into lipid molecules.
3.2. Melatonin Is Preferentially Located at Hydrophilic/Hydrophobic Membrane Interfaces
All biological cell membranes comprise amphipathic lipid molecules with hydrophilic heads and hydrophobic tails that naturally form bilayers with headgroups oriented towards an aqueous environment and tails facing each other
[462]. The melatonin molecule is uncharged in the entire pH range
[410] and, accordingly, in laboratory environment, the “hydrophobic” molecule dissolved poorly in water
[463] except when solubilized in pure aqueous medium by specific methodology that polarizes the pyrrole ring to facilitate hydrogen bonding of the N–H group
[464]. The unique ability to form strong H-bonds with hydrophilic lipid headgroups allowed nonpolar melatonin to be preferentially located at hydrophilic/hydrophobic interfaces, with complete solubility observed at the interfaces between polar and lipophilic nanodomains in reversed micelles
[320]. The presence of both hydrophilic and lipophilic moieties in melatonin facilitates the scavenging of both aqueous and lipophilic free radicals
[411], especially
•OH
[448] and
•OOH, the two most prevalent ROS responsible for the chain oxidation of unsaturated phospholipids
[465,466][465][466] in the membranes of cells and mitochondria
[467,468][467][468].
3.3. Melatonin Metabolite Free Radical Scavenging Cascades Rescue Cardiolipin from Hydroperoxyl Radicals (•OOH)
Lipid peroxidation, a physiological process in all aerobic cells
[469], is a cascading chain reaction that begins with the abstraction of allylic hydrogen from adjacent lipid molecules by free radicals such as
•OOH and
•OH and terminates with reactive aldehyde end products such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE)
[212,470,471,472,473][212][470][471][472][473]. Both
•OOH and
•OH are derived from ubiquitous superoxide radicals (O
2•−) generated from the one-electron reduction of oxygen (O
2) that may be catalyzed by nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) during respiratory bursts
[474] and/or electron leakage during mitochondrial electron transport
[403]. Due to its low rate constant values below ~102 L·mol
−1·s
−1 [475], O
2•− behaves more similarly to an unimpressive reductant (E°′(O
2/O
2•−) = −0.33 V) than an oxidant (E°′(O
2•−/H
2O
2) = 0.93 V)
[472,476,477,478][472][476][477][478] which reacts at a much slower pace with the tested phospholipids compared to
•OOH
[466,479][466][479]. Hydroperoxyl (
•OOH or HO
2•), also known as a perhydroxyl radical, is a chemically active, protonated form of superoxide radicals (O
2•−)
[480], engaged predominantly as intermediates for the disproportionation of O
2•− into hydrogen peroxide (H
2O
2) which then can further be transformed via Fenton’s/Haber–Weiss reactions
[481] into
•OH, possibly the most reactive and mobile species of oxygen that interacts with almost all molecules in cells
[212,481][212][481]. Even though at neutral pH
•OOH exists primarily as the less reactive O
2•−, where the ratio of protonated
•OOH to anionic O
2•− is ~130:1 (less than 1%),
•OOH can be a potent initiator of lipid peroxidation
[465,466][465][466].
When reacting with phospholipids, the advantageous free energy profile of −8.5 kJ/mol free energy minimum relative to the aqueous phase allowed
•OOH to accumulate at lipid headgroup membrane–water interface at concentration enhancement of over one order of magnitude
[295]. Multi-level atomistic simulations for interactions of
•OH,
•OOH, and H
2O
2 with polar headgroups of phospholipid bilayer revealed that all three species traveled deep into the water layer to reach phospholipid biomolecules, oxidizing hydrophilic headgroups before hydrophobic tails
[482], with
•OOH staying adsorbed for the longest duration at headgroup regions
[295]. The headgroup of CL is fully ionized as a dianion in the physiological pH range
[483], supporting its unique, optimal functionality as a “proton trap” that promotes mitochondrial respiratory enzyme activities
[484].
The strong negative curvature of cristae in the IMM is primarily sustained by the distinct molecular geometry of CL with its smaller, elongated, conical-shaped, double-phosphate dianonic headgroups that increase lateral pressure within the acyl chain regions and stabilize cylindrically curved, tubular cristae structures
[223,485,486][223][485][486]. In large unilamellar vesicles (LUVs) comprising similar lipid properties as the IMM, the addition of a typical concentration of 25% negatively charged, dianonic CL lowered pH at the membrane interface to ~3.9, compared to the bulk pH of 6.8 normally found in mitochondrial intermembrane space
[487] and 7.7 in the matrix space
[488]; in contrast, LUVs with mono-anionic lipids only reduced the pH to ~5.3 at the membrane interface
[487]. The reduced pH at the membrane interface from CL, linearly associated with increased proton (H
+) concentration (~700 to ~800)
[487], is the reason why ATP production is doubled in mitochondrial models with cristae compared to those without
[409]. At the same time, the increased H
+ concentration at membrane surfaces may cause accumulation of
•OOH, the protonated form of O
2•− [480].
•OOH remains adsorbed at polar headgroups longer than other ROS tested
[295]; therefore, a low pH at membrane interface that is favorable for enhanced ATP synthesis could also initiate peroxidation cascades. As such, even though the proper functioning of CL is prerequisite for optimal mitochondrial respiration and ATP production, peroxidation of CL in mitochondria is an inevitable, natural, physiological process that can deteriorate pathologically
[239,241,405,489,490,491,492,493,494,495,496,497,498][239][241][405][489][490][491][492][493][494][495][496][497][498] unless properly counterbalanced by the continuous synthesis
[420] and/or uptake of high levels of melatonin. Melatonin is known for its role in maintaining systemic energy homeostasis
[499]. In the mitochondria of brown and beige adipose tissue, CL biosynthesis is robustly induced upon cold exposure
[500,501][500][501] because CL can bind tightly to uncoupling protein 1 (UCP1), stabilizing its conformation and enhancing functionality
[502]. The ability of melatonin to protect CL from peroxidation may account for the increased thermogenic response in Zücker diabetic fatty (ZDF) rats via the restoration of UCP1 mRNA expression, increased mitochondrial mass and brown adipose tissue (BAT) weight, as well as enhanced mitochondrial OXPHOS activities in complex I and IV
[503].
3.4. Melatonin Antioxidant Cascades May Inhibit NLRP3 Prionoid-Like Aggregation in an ATP-Dependent Manner
Cardiolipin (CL) is a mitochondria signature lipid distinctly attracted to membrane lipid domains with strong negative curvatures, such as the apex of IMM cristae [226,228][226][228]. CL is often externalized to the outer mitochondrial membrane (OMM) upon mitochondrial distress from ROS attacks [258,259][258][259], whereas oxidized CL in OMM initiates apoptotic signaling processes [260] that can lead to opening of the mitochondrial permeability transition pore (mPTP) and the release of cytochrome c (Cyt c) [261,262][261][262]. Externalized CL, whether oxidized or not, becomes an essential signaling platform that binds and interacts with important mitophagic, autophagic, and inflammatory enzymes [259[259][263],263], including Beclin 1 [264], tBid, Bax [262[262][265],265], caspase-8 [266], and the NLR pyrin domain containing 3 (NLRP3) inflammasomes [267]. A major source of extremely inflammatory cytokines IL-1β and IL-18 [268], NLRP3 inflammasome is a phase-separated supramolecular complex that mediates immune responses upon the detection of cellular stress and dysfunction [269,270,271][269][270][271]. The activation of the NLRP3 inflammasome in macrophages is induced by oxidized phospholipids [272], whereas the docking of externalized CL to NLRP3 inflammasome primes its assembly and subsequent activation in mitochondria [267] as well as mitochondria-associated membranes (MAMs), a region comprising highly specialized proteins which is tethered to the endoplasmic reticulum (ER) [273,274][273][274].
Melatonin is a potent antioxidant that has been shown to inhibit CL peroxidation in mitochondria, preventing mPTP opening and Cyt c release
[301] by inhibiting peroxidation cascades initiated by specific ROS that accumulate in lipid headgroups at membrane–water interfaces
[295] (
Figure 1). The suppression of oxidative stress and lipid peroxidation may halt the externalization or oxidation of CL, effectively preventing potential pathological interactions with MLOs such as α-syn and the NLRP3 inflammasome. The interaction between pathological α-syn oligomers and externalized CL can result in increased ROS, lipid peroxidation, and mitochondrial dysfunction; therefore, it is not surprising that melatonin has been demonstrated to block α-syn fibril formation and oligomerization, decreasing cytotoxicity in primary neuronal cells
[302], as well as rescuing impaired mitochondrial respiration induced by α-syn in
Saccharomyces cerevisiae under ROS attack
[303]. The NLRP3 inflammasome must be primed by externalized CL upon ROS stimulation before activation
[258,267,273][258][267][273]. The regulation of the next phase where the NLRP3 inflammasome transitions into stable, prionoid-like complexes is mediated by DDX3X, one of the ATP-bound forms of DEAD-box RNA helicases responsible for the scaffolding of prionoid, self-oligomerizing specks known as apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC) which cannot be easily disassembled once they are formed
[304,305,306][304][305][306] (
Figure 2).
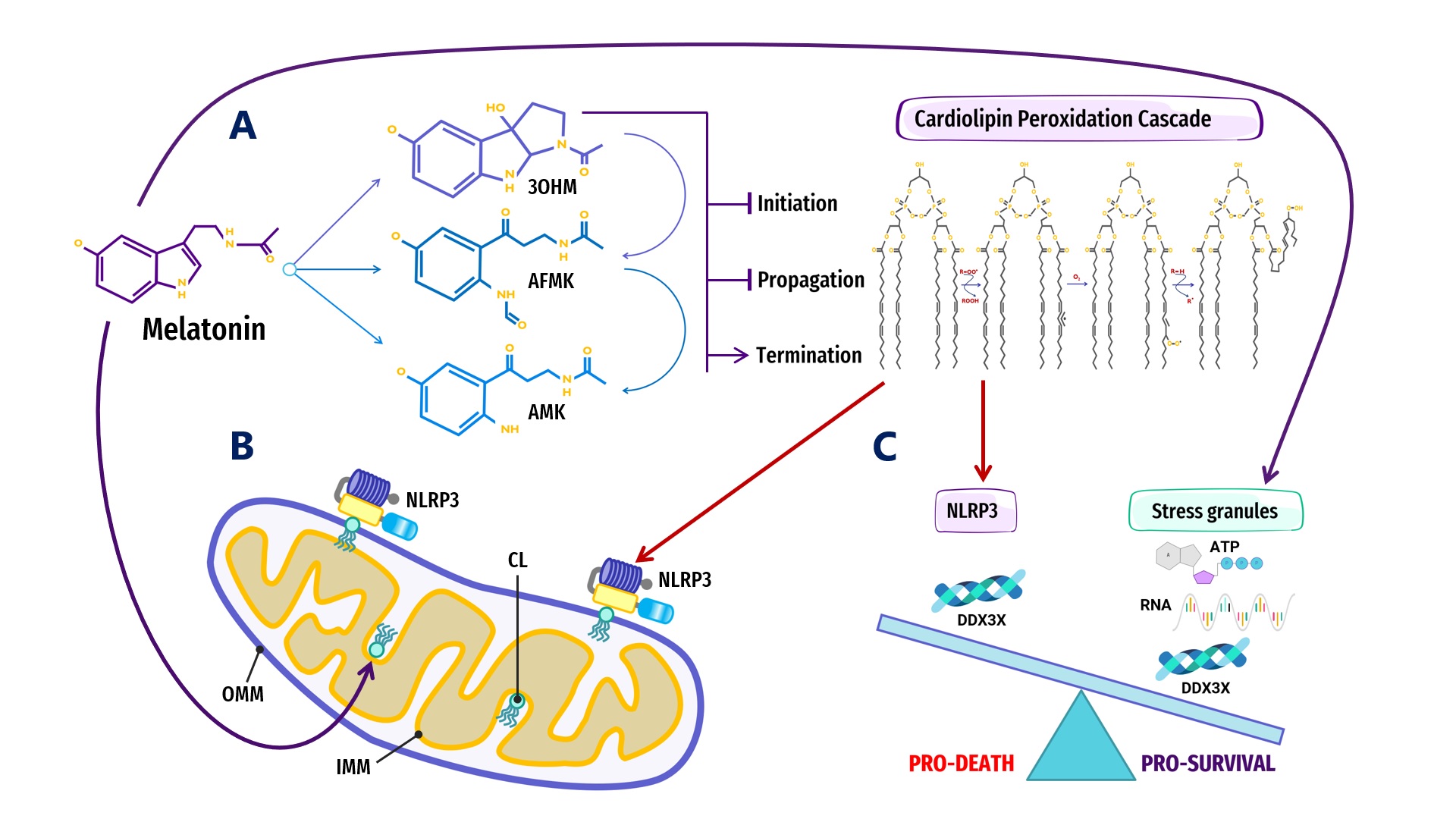 Figure 2.
Figure 2. Overview of melatonin regulation of NLRP3 inflammasome (NLRP3) formation, assembly and activation: (
A) Summary of melatonin and metabolite antioxidant cascade inhibiting the initiation and propagation of cardiolipin (CL) peroxidation, effectively terminating the CL peroxidation cascade; (
B) Oxidized CL is externalized from the cristae/inner mitochondrial membrane (IMM) to the outer mitochondrial membrane (OMM) where it docks and primes NLRP3 inflammasome assembly prior to activation in mitochondria; (
C) DDX3X, an ATP-dependent DEAD-box RNA helicase, is the mediator that selects the formation of “Pro-Survival” stress granules or the transition of the NLRP3 inflammasome into “Pro-Death”, stable, prionoid-like complexes. The successful formation of stress granules is also dependent upon the availability of ATP and RNA, both of which may be regulated by melatonin (See Abbreviations for additional acronyms).
ATP-dependent DEAD-box RNA helicases (DDXs) are ATPases that post-translationally regulate RNA-containing phase-separated organelles in prokaryotes and eukaryotes [307,308][307][308]. DDXs promote phase separation in their ATP-bound form, but can also release RNA and induce compartment turnover using ATP hydrolysis. Inhibition of DDX ATPase activity can disrupt the disassembly of physiological MLOs such as P-bodies and stress granules [69,309][69][309] (Figure 1). Phosphorylation is one of the most important PTMs that can control the assembly/disassembly of MLOs [608] as well as stabilize or destabilize MLOs including G bodies [512] and p53 [609]. Cells rely on phosphorylation as rapid, reversible responses to different stimuli by changing the physicochemical properties of proteins during phase separation multivalent interactions [79,538][79][538]. Phosphorylation establishes covalent bonds between phosphoryl and amino acid hydroxyl groups using the terminal phosphate group in ATP [610]. The ATP-dependent DEAD-box helicase [307] DDX3X responsible for initiating NLRP3 inflammasome aggregation is dependent upon phosphorylation-associated IFN promoter stimulation [304,310,615,616][304][310][615][616]. When the conserved, eukaryotic, integrated stress response (ISR) pathway is activated by external stress stimuli including hypoxia, nutrient deprivation, viral infections, as well as intrinsic ER stress [617], the phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2a) on Ser51 [618,619][618][619] triggers the formation of stress granules as adaptive homeostatic responses to promote survival and restore homeostasis [620,621,622,623][620][621][622][623]. It is presently unknown what prompts DDX3X to select the aggregation of pro-survival stress granules over pro-death NLRP3 inflammasomes or vice versa [304,310][304][310]. It would not be unreasonable to assume that an excessive oxidative local environment with pathological, enlarged lipid rafts (inflammarafts) [196,198][196][198] in membranes could exert a decisive influence over the selection process (Figure 2).
The activation of the NLRP3 inflammasome is now associated with major neurodegenerative disorders such as AD, PD and ALS, where positive correlations have been found to exist between NLRP3 levels and abnormal protein aggregations such as Aβ and α-Syn, whereas the inhibition of the NLRP3 pathway attenuates pathological protein aggregations
[311]. Melatonin inhibited NLRP3 inflammasome activation and reduced the aggregation of ASC specks in the mice hippocampus with major depressive disorder induced by inflammatory liposaccharides
[312]; melatonin also inhibited the formation of hypoxia-induced inflammasome protein complexes and reduced the aggregation of ASC specks in macrophages of Sugen/hypoxia pulmonary arterial hypertension (PAH) mouse models
[313]. Melatonin attenuated the progression of intervertebral disc degeneration in vitro and in vivo by reducing mitochondrial ROS products to inhibit NLRP3 inflammasome priming and activation, effectively terminating pro-inflammatory cytokine expression
[314]. The ability of melatonin to prevent the opening of mPTP and release of Cyt c
[301], inhibit NLRP3 inflammasome priming, activation, and ASC speck aggregation
[312[312][313],
313], block α-syn fibrillation
[302], and improve mitochondrial respiration
[303] could be directly related to its ability to stabilize nanoscopic lipid raft domains and suppress lipid peroxidation, which can alter the composition and molecular structures of lipid rafts.
During lipid peroxidation events, oxidized moieties were found to mainly reside close to the lipid headgroups forming hydrogen bonds with water. These oxidized lipids can perturb membrane bilayer structures and modify membrane properties, including decreasing the membrane fluidity
[318,353,354,355][318][353][354][355]. The preferential location of melatonin in bilayer lipid headgroups allows dynamic interactions that lead to reductions in bilayer thickness and increased bilayer fluidity
[338,341,356][338][341][356]. Eukaryotes and prokaryotes use ATPases localized in cell membranes and lipid raft domains to produce and release ATP energy
[122,127,136,152][122][127][136][152]; therefore, increased ATPase activities from enhanced membrane fluidity
[357,358][357][358] can impact how ATP interacts with phospholipids in bilayers
[216] and modulate the LLPS of MLOs formed at membrane surfaces
[45]. Moreover, lipid peroxidation is believed to be associated with the reduction in mitochondrial membrane fluidity during aging in animals
[359]. Membranes themselves can affect local protein concentrations
[360] where high-curvature lipids that form rafts may attract specific proteins that form aggregates to further enhance membrane curvature
[361,362,363,364][361][362][363][364]. Increasingly, neurodegenerative diseases such as AD are viewed as membrane disorders
[203]. The size of MLOs that aggregate at membrane surfaces can be tuned through PTMs such as phosphorylation, which is ATP-dependent
[365]. The amount of ATP available at membrane surfaces and cytosol drives the formation, tuning, and dissolution of MLOs, and is regulated by oxidative-stress-sensitive ion channels that reside in lipid rafts (
Figure 1).
3.5. Melatonin Maintains a High Cytosolic ATP:ADP Ratio through the Optimization of VDAC-CYB5R3 Redox Complexes in Lipid Rafts
Lipid rafts are phase-separated regions in lipid bilayers responsible for important biological functions including signal transduction [92,93][92][93] as well as the trafficking and sorting of proteins and lipids [94,95][94][95]. The fact that lipid rafts are also important redox signaling platforms that assemble, recruit, and activate redox regulatory multiprotein complex NADPH oxidase [182[182][366],366], and host the quintessential plasma membrane redox enzyme complex VDAC-CYB5R3 [367[367][368],368], emphasizes the relevance of melatonin as an antioxidant in the protection and stabilization of lipid raft domains.
Nanoscopic transient lipid raft domains in biological membranes are formed by phase separation in response to external stimuli [92,93,188][92][93][188]. Even though cells may alter lipid constituents to control the composition and size of lipid rafts [315], the propagation of molecular stress, lipid raft rattling dynamics and relaxation are some of the basic mechanisms underlying phase separation on the molecular level [195]. The presence of hydrophobic molecules such as melatonin can modulate viscoelastic dynamics through the accumulation and propagation of stress in lipid–lipid interactions [195,316][195][316]. Adding melatonin to membrane models led to a breakdown of out-of-phase membrane displacement patterns and the disruption of the vibrational landing platform of lipid biomolecules at the water–membrane interface, effectively slowing the permeation of ROS and other small molecules [195,317][195][317].
In 2005, melatonin was first observed to induce phase-separation in DPPC lipid bilayers [318]; recently, melatonin has been observed to modify lipid hydrocarbon chain order to promote phase separation in ternary membrane models [319]. Due to a preference to localize at membrane interfaces [320], melatonin can form strong hydrogen bonds with membrane lipid anionic headgroups that could significantly modulate lipid acyl chain flexibility and lipid dynamics [318]. Melatonin is able to directly interact with cholesterol [321] and displaced cholesterol due to competitive binding to lipid molecules, increasing disorder in the Ld phase to drive cholesterol into the ordered Lo phase [319]. These subtle changes in lipid nanodomains can profoundly affect amyloid processing at membrane sites. Aβ1–40 and Aβ1–42 peptides are known to interact strongly with negatively charged lipids by binding to anionic, negatively charged membranes [322,323,324,325,326][322][323][324][325][326]. Increasing cholesterol content lowered the surface charge of lipid membranes in saline solution from positive to negative [327]. Although cholesterol is an indispensable constituent of lipid rafts [92[92][162],162], its electrostatic properties altered interactions of charged or polar biomolecules on lipid membrane surfaces and attracted the targeted binding of Aβ deposits at lipid membranes [328,329,330,331][328][329][330][331].
Local variations in melatonin concentration also affected the re-ordering of lipids in membranes. At 0.5 mol% concentration, melatonin was documented to penetrate lipid bilayers to form fluid domains that enriched lipid membranes where melatonin molecules aligned parallel to phospholipid tails with the electron-dense regions slightly below hydrophilic headgroups; however, at 30 mol% concentration, melatonin molecules aligned parallel to the lipid bilayer, close to the headgroup regions where one melatonin molecule was associated with two lipid molecules to form an ordered, uniform, lateral membrane structure distributed evenly throughout the membrane model [341]. Variations in local concentration and conformational changes in melatonin molecules can directly impact the lipid phase transition, line tension, size, health, and functions of lipid rafts.
Present in all eukaryotes [369], CYB5R3 encodes for a NADH-cytochrome b5 reductase 3 flavoprotein that is engaged in the one-electron transfer from NADH to cytochrome b5 or plasma membrane coenzyme Q, producing NAD+ as a result [370,371][370][371]. The soluble isoform of CYB5R3 is exclusive to erythrocytes [372], whereas the membrane-bound isoform is anchored to MOM, ER, and plasma membrane lipid rafts [368,373,374][368][373][374]. Importantly, the OMM-bound CYB5R3 enzyme, ubiquitously expressed in all mammalian cells, is functionally attached to the voltage-dependent anion channel 1 (VDAC1), one of the most prevalent proteins located in the OMM [375,376][375][376].
Originally known as mitochondrial porin after its identification in yeast (1985) [377] and humans (1989) [378], VDAC was subsequently observed as a resident protein of lipid rafts in the plasma membranes of animal hearts, brains, and lungs [379] from different human cell lines, including epithelial cells, astrocytes, and neurons [380,381][380][381]. Aberrant lipid composition in neuronal lipid rafts disturbs physiological VDAC protein interactions that can affect the opening and closing of VDAC channels, resulting in oxidative stress and neuronal impairments prominent in most AD pathologies [380]. The force-from-lipid principle dictates that the opening and closing of membrane embedded channels can be propelled by the mechanical properties of surrounding lipids [382,383,384,385][382][383][384][385] and their composition. Changes to raft thickness, curvature and elasticity [291] as a result of lipid peroxidation can therefore affect physiological functions of the VDAC and CYB5R3 redox complex.
CYB5R3 enzymes form large redox centers in lipid rafts that enhance mitochondrial respiration rate and ATP production, albeit resulting in increased production of ROS [368,373,374][368][373][374]. Over stimulation and clustering of CYB5R3 induced oxidative stress-mediated apoptosis of cerebellar granule neurons [386]. Independent of respiratory chain activities, the ascorbate-dependent NADH: cytochrome c oxidoreductase oxidation of NADH at CYB5R3 centers in lipid rafts is also a major source of extracellular superoxide [376,387,388,389,390][376][387][388][389][390] that can initiate lipid peroxidation. In Wistar rats, the deregulation of CYB5R3 promptly triggers apoptosis due to the overproduction of superoxide anions at neuronal plasma membranes [368,387][368][387]. Excess NADH due to CYB5R3 redox dysfunction can close VDAC, suppressing OXPHOS and increasing glycolysis [376[376][391],391], whereas the opening of VDAC also elevates ROS from increased OXPHOS activities [41]. As the most abundant protein in the MOM, VDAC is regarded as a dynamic regulator of mitochondrial functions, interacting with over 100 proteins in health and disease [392]. VDAC opening is believed to globally control mitochondrial metabolism and ROS formation, modulating mitochondria and cellular bioenergetics [41,393][41][393]. Nevertheless, the question of whether apoptosis is associated with the opening [394] or closure [395,396][395][396] of VDAC has been highly debated [397], further emphasizing the important role of this protein in the regulation of cell life and death [392,398][392][398].
VDAC is the gatekeeper which controls the export of ATP out of mitochondria into cytosol and the import of essential respiratory substrates such as ADP and Pi into mitochondria [395,399][395][399]; therefore, VDAC opening may be instrumental in determining the fate of MLO formation, regulation, and dissolution. ATP is not only a biological hydrotrope capable of inhibiting protein LLPS and aggregation at high mM concentrations, but it has recently been observed to act as a universal and specific regulator of IDRs capable of altering physicochemical properties, conformation dynamics, assembly, and the aggregation of MLOs [45]. Not only is the preservation of lipid raft structure and composition essential for maintaining specific ion channel properties [380], the amount of cytosolic ATP is dependent upon mitochondrial synthesis and the integrity of CL enriched raft-like lipid domains in mitochondria [367,400,401,402][367][400][401][402].
The mitochondrial electron transport chain is a major ROS-generating site where complex III and mitochondrial glycerol 3-phosphate dehydrogenase can produce large amounts of redox signaling molecules such as superoxide and hydrogen peroxide to the external side of the IMM as well as the matrix
[403,404][403][404]. Bis-allylic methylenes and abundant double-bonds in CL lipid chains are vulnerable targets of ROS attacks
[239,405,406,407][239][405][406][407]; therefore, the lipid monolayer leaflets facing the crista lumen enriched in CL in mitochondria
[228] may be subject to intense peroxidation events. Peroxidized CL could not support mitochondrial OXPHOS enzyme activities
[239[239][408],
408], leading to the depletion of ATP
[409] that can potentiate and exacerbate the aggregation of pathological MLOs.
Melatonin is an ancient, potent antioxidant that protects lipid nanodomains from peroxidation caused by excess oxidative stress. The addition of micromolar concentrations of melatonin to rat heart mitochondria dramatically inhibited CL oxidation by tert-Butylhydroperoxide (t-BuOOH), a peroxidation promoting peroxide, reversing cytochrome c release, matrix swelling, and proton motive force (ΔΨ) collapse in treated cells
[301]. The melatonin molecule is uncharged in the entire pH range
[410] and contains both hydrophilic and lipophilic moieties that support its easy accumulation in all internal membranes of cells as well as other hydrophobic sites
[411,412][411][412]. The exogenous supplementation of melatonin in rodents results in dose-dependent increases in all subcellular compartments, with lipid membranes exhibiting 10-fold increases compared to mitochondria
[413]. The presence of both hydrophilic and lipophilic moieties in melatonin not only facilitates the efficacious scavenging of both aqueous and lipophilic free radicals
[411], but also places the molecule in a unique position during evolution to protect membrane lipids from oxidative damage and potentially regulate MLOs that form at membrane surfaces in an ATP-dependent manner (
Figure 1).
Melatonin protects the functionality of the VDAC–CYB5R3 complex by reducing oxidative stress, lowering ROS that may induce lipid peroxidation, which can alter raft composition, thickness, curvature and elasticity
[291] that may impact VDAC ion-channel opening/closure according to the force-from-lipid principle
[382,383,384,385][382][383][384][385]. VDAC expressed in the plasma membranes of HT22 mouse hippocampal neuronal cells were quiescent under control conditions with normal ATP and an absence of apoptotic signals. Serum deprivation increased ROS and induced VDAC opening in the plasma membranes of hippocampal HT22 cells, resulting in mitochondrial dysfunction and increased apoptosis and autophagy. HT22 cells pre-loaded with 200 μM melatonin prior to serum deprivation did not exhibit VDAC activities. In the same manner, the addition of 4 mM ATP blocked the activation of VDAC channels
[834],
with the implication that melatonin was able to maintain optimal VDAC functioning in an ATP-dependent manner.
3.6. Melatonin May Regulate Glycolytic G Bodies by Increasing ATP
The theoretical maximum of ATP calculated from simultaneous measurements of extracellular acidification and oxygen consumption indicated that OXPHOS ATP production was close to or more than 16 times above glycolysis, at 31.45 ATP/glucose (maximum total yield 33.45) and 2 ATP/glucose, respectively
[822]. As early as 2002, melatonin was found to increase mitochondria OXPHOS activity and elevate the production of ATP
[504]. Recent experimental and theoretical studies have presented different mechanisms explaining how melatonin may function as a glycolytic, such as stimulating the SIRT3/PDH axis in vitro to reverse the Warburg phenotype in lung cancer cells
[505], converting cells to a healthy phenotype by inhibiting hypoxia-inducible factor-1α to encourage OXPHOS over glycolysis induced by hypoxic conditions
[506], downregulating pyruvate dehydrogenase kinase (PDK) to increase acetyl CoA synthesis
[507[507][508],
508], or elevating α-ketoglutarate (α-KG) levels in macrophages to promote M2 polarization that favors OXPHOS over glycolysis
[509,510][509][510].
Interestingly, in
Saccharomyces cerevisiae and human hepatocarcinoma cells challenged with hypoxic stress, the non-canonical RNA-binding proteins in glycolytic enzymes have been observed to promote phase separation
[511] that facilitate and maintain the assembly of glycolysis enzymes into cytoplasmic, membraneless glycolytic G bodies that increased glycolytic output during hypoxia
[512]. Melatonin is able to increase ATP concentration in cells
[503,504,505][503][504][505]; therefore, the switch between OXPHOS and glycolysis could possibly be part of the effect where high ATP concentration dissolves MLO aggregations. Molecular dynamics simulation experiments revealed that the propensity for self-aggregation enhanced the role of ATP as a hydrotrope, preferentially binding to polymers to unfold hydrophobic macromolecules and disrupting the aggregation process of hydrophobic assemblies via the introduction of charges to the macromolecules
[513]. These results may explain previous observations where a high cytosolic ATP:ADP ratio readily suppressed glycolysis, whereas the closure of VDAC channels resulting in lower ATP:ADP ratios in cytosol activated glycolysis in vitro
[514]. Alterations to the glycolytic pathways are often observed during the early stages of neurodegenerative diseases where mitochondrial dysfunction and reduced ATP levels may contribute to protein aggregation
[515]. Increasingly, the pathogenic aggregation of MLOs such as stress granules, p53, FUS, TDP-43, and tau exhibiting dysregulated LLPS is believed to play a major part in the development of neurodegeneration and cancer
[12,516,517,518][12][516][517][518].
The relationship between melatonin and ATP is likely an ancient one that might date as far back as ~4 billion years ago when a proposed gene duplication event at ~3.5 Ga involving CP43 and CP47, enzymes unique to photosystem II (PSII), marked the beginning of water oxidation
[431]. Regulation of the synthesis and degradation of the evolutionarily conserved PSII D1 reaction center is mediated by post-translational RNA modulations
[1118,1119,1120][1118][1119][1120] and the presence of ATP
[1121] in a light-dependent manner, where synthesis and/or degradation is induced by light but ceased in the dark. Unlike animals
[1122], melatonin in plants is increased by the presence of light
[1123[1123][1124],
1124], and treatment with melatonin enhanced the synthesis of PSII D1 reaction centers in tomato seedlings under salt stress
[1125]. Cyanobacteria, the only known prokaryote capable of water oxidation
[431] which also produces melatonin
[421[421][422],
422], has recently been shown to exhibit circadian rhythm in the formation and dissolution of MLOs that remained soluble during daylight, but became reversible, insoluble condensates at night in an ATP-dependent manner
[432]; therefore, it is not unreasonable to hypothesize that the relationship between melatonin, MLOs, and ATP was already in existence at ~3.5 Ga. The presence of melatonin in primitive unicellular organisms including
Rhodospirillum rubrum and cyanobacteria, precursors to mitochondria and chloroplasts, respectively
[415[415][423][424][425],
423,424,425], may have conferred protection against endogenous and exogenous oxidative stress that could readily damage macromolecules and disrupt ATP production at membrane lipid domains
[421,426,427][421][426][427]. This unique feature implies that melatonin may have an intrinsic modulatory effect over phase separation, not only in early but present-day organisms (
Figure 1).
4. Conclusions
The physiological and pathological functions of biomolecular condensates in health and disease may be shaped by powerful, complex, interdependent relationships between membraneless organelles, membranes/lipid rafts, ATP, and most of all, stress and its timely resolution. Melatonin’s intimate association with each of these decisive influencers may position the potent, ancient antioxidant as an important mediator of the phase separation of condensates in health and disease via principal ATP-dependent post-translational mechanisms and regulation of ATP levels in mitochondria and cytoplasm (Figure 1). This novel theoretical review highlights the important connections between melatonin and ATP in the regulation of biomolecular condensates with the intention to spur further research interest and exploration in the full, multi-faceted potential of melatonin that may provide solutions and answers to existing and future challenges and questions in this exciting and promising field of study.
 Figure 1. Schematic illustrating the regulation of biomolecular condensates by melatonin represented through observations reported in antioxidant protection against lipid peroxidation to maintain membrane/lipid raft composition/stability that serves to maintain adequate ATP levels in all cellular compartments to fuel, support, and regulate post-translational/m6A modifications that may fine-tune RNA dynamics in the assembly and disassembly of MLOs to prevent pathological aggregations in neurodegenerative disorders. LLPS: liquid–liquid phase separation; DDX: Dead-box RNA helicase; m6A: N6-methyladenosine; MLO: membraneless organelle; RBP: RNA-binding protein; RNP: ribonucleoprotein; PTM: post-translational modification (See Abbreviations for additional acronyms).
Figure 1. Schematic illustrating the regulation of biomolecular condensates by melatonin represented through observations reported in antioxidant protection against lipid peroxidation to maintain membrane/lipid raft composition/stability that serves to maintain adequate ATP levels in all cellular compartments to fuel, support, and regulate post-translational/m6A modifications that may fine-tune RNA dynamics in the assembly and disassembly of MLOs to prevent pathological aggregations in neurodegenerative disorders. LLPS: liquid–liquid phase separation; DDX: Dead-box RNA helicase; m6A: N6-methyladenosine; MLO: membraneless organelle; RBP: RNA-binding protein; RNP: ribonucleoprotein; PTM: post-translational modification (See Abbreviations for additional acronyms). Figure 2. Overview of melatonin regulation of NLRP3 inflammasome (NLRP3) formation, assembly and activation: (A) Summary of melatonin and metabolite antioxidant cascade inhibiting the initiation and propagation of cardiolipin (CL) peroxidation, effectively terminating the CL peroxidation cascade; (B) Oxidized CL is externalized from the cristae/inner mitochondrial membrane (IMM) to the outer mitochondrial membrane (OMM) where it docks and primes NLRP3 inflammasome assembly prior to activation in mitochondria; (C) DDX3X, an ATP-dependent DEAD-box RNA helicase, is the mediator that selects the formation of “Pro-Survival” stress granules or the transition of the NLRP3 inflammasome into “Pro-Death”, stable, prionoid-like complexes. The successful formation of stress granules is also dependent upon the availability of ATP and RNA, both of which may be regulated by melatonin (See Abbreviations for additional acronyms).
Figure 2. Overview of melatonin regulation of NLRP3 inflammasome (NLRP3) formation, assembly and activation: (A) Summary of melatonin and metabolite antioxidant cascade inhibiting the initiation and propagation of cardiolipin (CL) peroxidation, effectively terminating the CL peroxidation cascade; (B) Oxidized CL is externalized from the cristae/inner mitochondrial membrane (IMM) to the outer mitochondrial membrane (OMM) where it docks and primes NLRP3 inflammasome assembly prior to activation in mitochondria; (C) DDX3X, an ATP-dependent DEAD-box RNA helicase, is the mediator that selects the formation of “Pro-Survival” stress granules or the transition of the NLRP3 inflammasome into “Pro-Death”, stable, prionoid-like complexes. The successful formation of stress granules is also dependent upon the availability of ATP and RNA, both of which may be regulated by melatonin (See Abbreviations for additional acronyms).