Molecular imprinting is a technique for creating artificial recognition sites on polymer matrices that complement the template in terms of size, shape, and spatial arrangement of functional groups. The main advantage of Molecularly Imprinted Polymers (MIP) as the polymer for use with a molecular imprinting technique is that they have high selectivity and affinity for the target molecules used in the molding process.
1. Introduction
Molecular imprinting is a technique for creating artificial recognition sites on polymer matrices that complement the template in terms of size, shape, and spatial arrangement of functional groups. This molecular imprinting technique uses target molecules in a synthetic polymer matrix by selectively binding
[1][2][1,2]. Recently, molecular imprinting technology has been used to create biometric surfaces in biosensors. There are many molecular imprinting technologies, including bulk printing, surface printing, and epitope printing.
In the bulk printing method, the template molecules are printed on the entire polymer matrix, and at the end of the method, the template needs to be removed from the polymer. The polymer produced in this method is large or bulk, so grinding must be carried out on the polymer to obtain template-specific binding sites on the polymer
[1]. Due to the thick morphology of the polymer in the bulk printing, it causes low access for the target molecule to bind to its specific site. Therefore, another method was developed to overcome this limitation, namely the surface printing method and the epitope printing method.
In surface printing, the removal of template molecules will result in specific binding sites on the polymer surface
[3]. The binding site on the polymer surface causes this type of polymer to provide greater access to the binding target molecule than bulk imprinting
[1]. This technique has been widely used for various types of analytes such as proteins
[4], cells
[5], and micro-organisms
[6]. While in epitope printing, the target molecule is a protein and uses only a small portion or fragment of the macromolecule is printed to represent the whole molecule (epitope) as a template
[1]. In this method, the peptide epitope is covalently bonded to the silicon surface where the monomer is polymerized. In epitope printing, more specific and strong interactions can be obtained. The polymer has the ability to recognize templates as well as whole proteins very well
[7].
Molecularly Imprinted Polymer (MIP), which contains specific bonds between template molecules and polymers
[8], is an example of materials that use molecular imprinting techniques. MIP is a unique recognition system resulting from templates and functional monomers that are polymerized, enabling molecular recognition utilizing principles similar to those underlying the action of enzymes and their substrates
[9][10][9,10]. The main advantage of MIP is high selectivity and affinity for the target molecules used in the molding process. Compared to biological systems such as proteins and nucleic acids, imprinted polymers have higher physical strength, high temperature, pressure resistance, and inertia to acids, alkalis, metal ions, and organic solvents. In addition, the synthesis cost is low, the storage life of the polymer can be very high, and the recognition capability can be maintained for several years at room temperature.
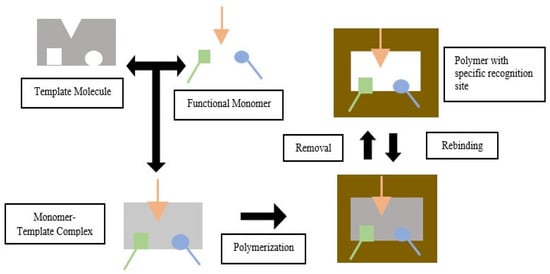
Templates, functional monomers, solvent, initiator, and cross-linker are MIP components
[11]. MIP is based on the formation of complexes between analytes (templates) and monomers of functional compounds. In the presence of excess cross-linking agents, three-dimensional polymers are formed
[12]. In the process of making MIP, the selection of the constituent components will affect the performance of the resulting imprinted polymer. A functional monomer is preferred over an ordinary monomer because a functional monomer contains a Y functional group that can interact with template molecule via hydrogen bonding, dipole-dipole, and ionic interaction to produce a template-monomer complex. The complex is then fixed in the presence of a large excess of a cross-linking agent, and a three-dimensional polymer network is formed. After the polymerization process, template molecules are removed from the polymer using a solvent, resulting in selective complementary polymer-template bonds
[11][13][11,13]. The scheme of the molecular imprinting process can be seen in
Figure 1.
Figure 1. Molecular Imprinting Process (green, orange and blue arrow indicated different type of functional groups that could be appeared in the functional monomer).
2. MIP Application
2.1. Environmental Monitoring
The increasing contaminants in the environmental water is an alarming issue
[14][18]. Environmental monitoring can be carried out on environmentally hazardous materials such as dyes
[15][19], persistent organic pollutants
[16][20], pesticide residues
[17][21], mycotoxins
[18][22], heavy metals, and antibiotics
[19][23]. The presence of these contaminants can cause harmful effects on public health, either directly or indirectly
[20][24]. Environmental matrices that can be polluted by pollutants include air, soil, atmosphere, sediment, and flora and fauna
[21][22][25,26]. The pre-treatment sample aims to eliminate matrix interference so that only the target analyte is obtained
[23][27]. MIP is commonly used as a sorbent in pre-treatment samples in samples with complex matrices because of its selected properties compared to conventional sorbents
[22][26].
One of the applications was presented by Song et al.
[24][28] that analyzed ten macrolide drugs (spiramycin, clarithromycin, erythromycin, tulathromycin, midecamycin, roxithromycin, josamycin, kitasamycin, tilmicosin, and azithromycin) in environmental water. MIP with functional monomer methacrylic acid and tylosin as a template was used as a sorbent in the molecular imprinted solid-phase extraction (MI-SPE) pre-treatment technique and then analyzed using the LC-MS/MS method. As a result, MI-SPE showed a high recognition ability of macrolides. The mean percentage recovery of macrolides at four spiked concentration levels was 62.6–100.9%, with intra-day and inter-day relative standard deviations below 12.6%.
2.2. Food Analysis
Contaminants from the environment can enter the body through food or drinking water and have the potential to cause harmful effects on health
[25][29]. For example, insecticide and herbicide contaminants used in agriculture include fruits, vegetables, and cereals. Effective analytical methods and technologies are needed to ensure food safety. Food is a complex matrix sample, so MIP can be used as a sorbent in the analysis process
[26][30].
Garcia et al.
[27][31] assessed dimethoate spiked in olive oil using core-shell magnetic-photonic dual responsive molecularly imprinted polymers (magnetic-photonic DR-MIP) as sorbent. Sample preparation using a DR-MIP-based sorbent is superior to the MI-SPE technique in terms of the procedure and minimized processing time. As a result, DR-MIP was declared a promising sorbent for the analysis of spiked dimethoate olive oil in sample preparation because it produced a high percentage recovery of 83.5% ± 0.3% with a low detection limit of 0.03 μg/mL
[27][31].
Besides olive oil, MIP is also used for the analysis of macrolide antibiotics in honey, milk, and drinking water samples
[28][32], porcine serum albumin in raw meat extract
[29][33], lincomycin residue in pasteurized milk
[30][34], triazine pesticides on cereals
[31][35], fluoroquinolones in fish samples
[32][36], and so on.
2.3. Biomedical Diagnostic
In biomedical applications, MIP is known as an artificial receptor that has basic capabilities like natural receptors, one of which is the ability to recognize cells
[33][37]. Compared to natural receptors, MIP has advantages such as high affinity and selectivity, more temperature stability, rapid preparation, and low costs
[34][38]. MIPs are designed to have specific recognition sites for target molecules such as antibodies and enzymes
[34][35][38,39]. In the immune system, antibodies must be specific in recognizing certain antigens. MIP as an alternative to antibodies is often used as an analytical method for diagnostic purposes
[36][40], such as breast cancer diagnostics
[37][41], cardiovascular disease
[38][42], and dengue fever
[39][43]. Therefore, MIP acts as a biomarker
[40][44] because it is able to show certain physical characteristics or measurable biologically generated changes in the body associated with certain diseases or health conditions
[41][45].
2.4. Drug Delivery
Drug-delivery systems (DDS) are a method of delivering drugs to the desired place before drug release and absorption so as to increase the pharmacological activity of the drug and achieve the desired therapeutic effect
[42][43][46,47]. DDS must be able to control the amount and speed of drug release
[42][46]. MIP as a DDS agent offers several advantages, namely long shelf life, easy preparation, high chemical, and physical stability, and low cost
[35][39].
Suksuwan et al.
[44][48] evaluated the ability of drug delivery to cancer cells using an enantioselective receptor for (R)-thalidomide enantiomer designed in nanoparticles using methacrylic acid, a fluorescently active 2,6-bis(acrylamido)pyridine and N,N
0-methylene-bis-acrylamide, via both a covalent approach and a physical approach. The results of his research show that MIP nanoparticles, through a physical approach, have the potential to make effective drugs to attack multidrug-resistant cells with the right temperature at the target location.
3. Choosing Right Component for MIP
Many factors can influence the success of making molecularly imprinted polymers. These factors include the properties of monomers, cross-linkers, and solvents
[45][46][47][17,49,50]. The properties of monomers, cross-linker, and solvents will affect the morphology and size of the polymer formed and template-monomer interactions.
3.1. Functional Monomers
Functional monomers are one of the components of MIP whose properties will influence the success of making a MIP. Functional monomers are an essential factor for binding interactions in molecular imprinting technology
[48][51]. The functional monomer will influence the binding site affinity of a MIP
[49][52]. In the MIP pre-polymerization, the functional monomers are going to interact with the template molecules
[11]. The formation of a stable template-monomer complex is critical to the success of MIP
[50][51][53,54]. It is commonly accepted that stronger interaction between the template and functional monomer will result in a more stable template-monomer complex prior to polymerization, and consequently, the better imprinting efficiency of the polymer resulting will be
[52][53][54][55,56,57]. Monomers are positioned spatially around the template, and due to cross-linking, the position of the individual repeat units that interact with template molecules will become fixed. Polymer microporous matrix possessing microcavities will be formed with a three-dimensional structure complementary to that of the template
[11][55][11,58].
Research conducted by Zhong M. et al.
[54][57] on the preparation of magnetic molecularly imprinted polymers for isolation of chelerythrine (CHE) showed that stable complex between CHE as a template (T) and MAA as a functional monomer (FM) in a 1:4 ratio gave the lowest binding energy in computational calculation compared to other ratios (1:1; 1:2; 1:3; and 1:5). The lowest binding energy indicated the highest complex stability. When the imprinting ratio (template:monomer) was 1:5, the excess monomers would increase their own association. At the same time, the spatial structure of the complex was unstable due to the space steric hindrance between the template and the functional monomer molecules. The result of the wet laboratory experiment also showed that the ratio (T:FM = 1:4) gave higher selectivity and adsorption capacity for template molecules toward analogous compounds.
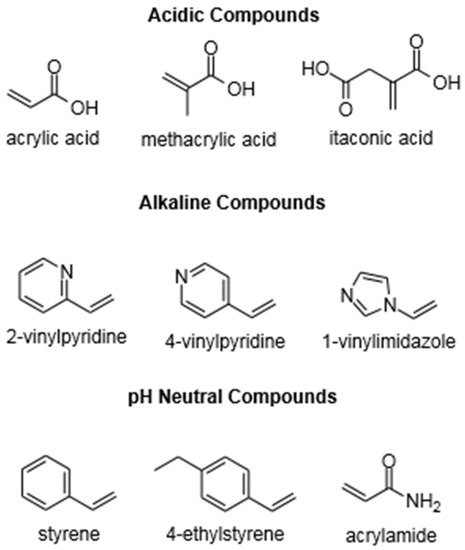
Three types of functional monomers are most commonly used: (i) acidic compounds, such as methacrylic acid, (ii) alkaline compounds, such as 4-vinyl pyridine, and (iii) pH neutral compounds, such as styrene
[56][59]. Some typical functional monomers are listed in
Figure 2.
Figure 2.
Chemical Structure of Functional Monomers.
A sufficiently high number of functional monomers is required to ensure a high binding capacity for the target molecule
[57][60]. If the amount of monomer is too large, it usually results in a more non-specific interaction site
[47][50]. The use of correct proportions of monomers and templates in the pre-polymerization process will lead to the formation of many high-affinity sites
[57][60]. The effect of monomer amount on MIP was studied by Zhao et al.
[58][61]. They studied the effect of amount variation of methyl methacrylate (MMA) as a functional monomer on the adsorption performance of a Solasenol MIP (SSO-MIP). Their research proved that the adsorption performance of a MIP would reach its highest point at a certain monomer concentration, and increasing the concentration after reaching the optimum point caused a decreased adsorption capacity value (Q). The highest point of SSO-MIP adsorption capacity, 43 mg/g, occurred when the MMA concentration was 0.2 mmol. When the MMA concentration was increased to 0.25 mmol, the adsorption capacity decreased to 41 mg/g, as well as at concentrations of 0.30 mmol (Q = 37 mg/g) and 0.35 mmol (Q = 36 mg/g)
[58][61].
The interaction between template and functional monomer must be interdependent, i.e., based on the nature of the template/analyte and its functional monomer (acid analyte: alkaline functional monomers; alkaline analyte: functional acid monomer)
[59][62], like the study of Xu et al.
[60][63] who synthesized MIP Hexamethylenetetramine. Hexamethylenetetramine is an alkaline compound, and they used methacrylic acid as the monomer. Meanwhile, in another study by Zunngu et al.
[61][64], they succeeded in synthesizing MIP with ketoprofen (acid) as a template and using 2-vinyl pyridine (alkaline) as a monomer.
The choice of functional monomer in MIP synthesis should also be based on the interactions formed by the template based on the functional groups in the selected template. Like the study of Barros et al.
[47][50], who synthesized MIP using hydrochlorothiazide as a template. Hydrochlorothiazide has three interaction sites, one site on the thiazide ring and two sites on the sulfonamide group. These groups can form hydrogen bonds with carboxyl, hydroxyl, amine, and even amide groups. Therefore, various monomers (methacrylic acid, allylamine, 4-vinyl pyridine, methacrylamide, acrylamide, acrylic acid, and 2-(trifluoromethyl)acrylic acid) were investigated, which may interact through hydrogen bonding with hydrochlorothiazide. The choice of monomer is based on the interaction energy formed between the monomer and hydrochlorothiazide through computational simulations. It is known that methacrylic acid is the best monomer for synthesizing MIP hydrochlorothiazide because it has the highest interaction energy compared to other monomers
[47][50].
3.2. Cross-Linker
The cross-linker, as a reagent of MIP, has a significant role in the formation of MIP. The role of the cross-linker is to participate in the formation of the physical characteristics of polymers in MIP. The physical characteristics of the polymer affected by the presence of the cross-linker are the morphological characteristics of the absorbent structure, three-dimensional structure, optimal stiffness, and the durability of the MIP
[56][62][59,65].
There are several important factors in the use of cross-linkers, including the type and number of cross-linkers used for polymerization. The higher number of cross-linkers makes it possible to obtain a stable porous material
[45][17]. A study was carried out by Zhao et al.
[58][61] to see the effect of cross-linker amount on adsorption performances of a MIP. Their research proved that the adsorption performance of a MIP would increase to a certain concentration of cross-linker. After reaching optimum conditions, the further addition of the cross-linker will not increase the adsorption performance; it will even decrease the adsorption performance. The chemical compounds most often used as cross-linkers are trimethylolpropane trimethacrylate (TRIM), divinylbenzene (DVB), and ethylene glycol dimethacrylate (EGDMA)
[56][63][59,66].
The presence of a cross-linker also affects the morphology of MIP that will affect the binding capacity of MIP. Studies conducted by Holland et al.
[64][67] and Rosengren et al.
[65][68] showed similar results that lower cross-linker concentrations provide greater binding capacity. This phenomenon occurs because the mean pore diameter decreased with increasing EGDMA, which may have resulted in a greater sieving ability of these polymers, which leads to lower overall capacity. Several studies have shown a comparison of the cross-linkers commonly used in MIP. Esfandyari-Manesh et al.
[63][66] compared the use of TRIM and EGDMA in MIP with methacrylic acid (MAA) as functional monomer and carbamazepine (CBZ) as the template. Their study showed that MIP using TRIM (MAA-TRIM) as a cross-linker showed better binding capacity and had more carbamazepine recognition sites compared to MAA-EGDMA, with MAA-TRIM binding capacity > 300 (around 300–500) micrograms CBZ while MAA-EGDMA binding capacity was around 300 micrograms CBZ, both measured at 180 min. Another study from Pangkamta et al.
[66][69] also, MIP with TRIM as a cross-linker showed a higher binding capacity percentage of 45–50% than MIP with EGDMA as cross-linker, which only shows 40–45%. The structure of TRIM and EGDMA possibly influences this. In the TRIM molecule, three-branched chains contain three vinyl groups, making TRIM more preferable in the polymerization process and resulting in more rigid complementary recognition sites for the template than the EGDMA molecule only has two vinyl groups. The contribution of the cross-linker, of course, also depends on other components, such as using the suitable functional monomer for the template or selecting the right solvent in the polymerization process
[63][66][66,69].
3.3. Solvents
Solvents act as a medium for reactions and have a significant effect on template-monomer interactions, which are certain components that must be considered to make a MIP. Solvents in the making of MIP must interact and dissolve all the starting materials but should not interfere too much during the polymerization reaction process
[57][60]. When a high solvation value is used in the synthesis process of a MIP, the solvent will protect the molecular interaction site and weaken the strong interaction between the monomer and template, thus making the molecular recognition ability of the MIP relatively poor
[67][14]. A study by Dong et al.
[68][70] examined the effect of solvents on the adsorption selectivity of MIP with theophylline (THO) as a template and MAA as a functional monomer. They compared three solvents, namely chloroform, tetrahydrofuran (THF), and dimethyl sulfoxide (DMSO), and found that DMSO had the highest affinity for THO and MAA but had the lowest imprinting factor (IF), 1.0533, compared to that of THF (IF = 1.1076) and chloroform (IF = 3.3197). The Imprinting Factor (IF) value shows a particular analyte’s distribution ratio on the imprinted polymer as well as under the same conditions as the non-imprinted polymer (NIP). An IF value larger than one indicates good imprinting
[69][71]. MIP with chloroform as solvent has the highest imprinting factor value because it provides the weakest interference on the template-monomer interaction, which results in the formation of the strongest hydrogen bonds between them
[68][70].
MIP is usually synthesized in the organic solvent to increase the hydrogen bonding and electrostatic interactions between the template and monomer
[70][72]. The polarity of the solvent greatly influences the template-monomer interactions. Less polar solvents promote the formation of the template-monomer functional complex, whereas more polar solvents interfere with the interactions in the template-monomer functional complex that form
[45][17]. The statement is supported by the results of research conducted by Song et al.
[71][73] on the effect of porogenic solvent in the manufacture of MIP for quercetin. They used four organic solvents with different polarities. The solvents are 1,4-dioxane, tetrahydrofuran (THF), acetone, and acetonitrile, from lowest to highest polarity. MIP with THF as the solvent showed a higher IF value of 1.2 compared to MIP using other solvents: 1,4-dioxane (IF = 1.05), acetone (IF = 1.07), and acetonitrile (IF = 1.03). This clearly shows that the medium polar solvent (THF) provides a better imprinting factor and the polar solvent (acetonitrile) provides the worst imprinting factor. Using a medium polar solvent (THF) will form an optimum interaction between the template and monomer and develop a uniform printing site on the MIP. When using a polar solvent (acetonitrile), the solvent interacts strongly with quercetin (template) and acrylamide (functional monomer), making it difficult for them to interact with each other. While using a less polar solvent (1,4-dioxane), a strong interaction will be formed between the template-monomer, but because the polymer’s solubility in the less polar solvent is low, the MIP formed will quickly form a precipitate
[71][73].
4. Template-Monomer Interaction
A study has shown that physical properties and recognition of MIP depend on the successful interaction between template and monomer. This process occurs in the pre-polymerization stage
[52][55]. The selected functional monomer will interact with the template, producing a stable template-monomer complex
[45][17]. Therefore, functional monomers and templates must complement each other
[56][59].
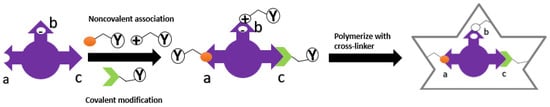
There are currently two strategies used in MIP technology based on the nature of the template-monomer interaction. Two types of molecular imprinting strategies have been set by covalent or non-covalent interactions between the template and functional monomer
[72][74]. An example of interaction can be seen in
Figure 3. These two strategies are:
Figure 3. Type of interaction between template and monomer: (
a) Noncovalent (nonionic) (
b) noncovalent (electrostatic/ionic) (
c) covalent.
1. Self-assembly approach, which uses non-covalent bonds between monomer and template, such as hydrogen bonds, Van der Waals forces, ionic or hydrophobic interactions, and others
[11]. The functional monomers are regularly positioned around the template molecules during the self-assembling process due to different exchanges
[73][75];
2. Pre-organized approach, which uses reversible covalent bonds between the functional monomer and template. This strategy will reduce non-specific sites on MIP
[11][74][11,76].
The technique most widely used in the manufacture of MIP is the self-assembly approach. In this technique, template-monomer complexes are formed in situ by non-covalent interaction
[75][77]. Hydrogen bond, hydrophobic, and electrostatic interactions are the most widely used bonds for manufacturing MIP due to their excellent adaptability
[62][65].
MIP manufacturing techniques using covalent and non-covalent bonds have their respective advantages and disadvantages, as listed in Table 1.
Table 1. Comparison Between Covalent and Non-Covalent Imprinting Techniques.
|
| Imprinting Type |
|
| Covalent |
|
| Non-Covalent |
|
| References |
|
|
| Interaction |
|
|
-
Reversible condensation reactions (ketal, acetal, esters, boronate, Schiff’s bases)
|
|
| |
|
[45][76][77]
|
[17,78,79]
|
|
| Advantage |
|
|
-
More durable and rigid types of interactions
-
The template-monomer complex is usually stable during the polymerization process
-
Forming a polymer with a more homogeneous binding cavity
|
|
|
-
Flexible, fast, and straightforward binding interactions
-
Easy template molecule removal
-
Easy template-monomer complex preparation
-
Resulting in a MIP with high-affinity binding, greater affinity, and selectivity to the site
|
|
[45][46][48]76][78]
|
[17,49,51[,78,80]
|
|
| Disadvantage |
|
| |
|
| |
|
[45][77]
|
[17,79]
|
5. Analysis of Template-Monomer Functional Interactions
Intermolecular interactions between molecular templates and functional monomers will affect the selectivity and affinity of MIP
[79][82]. These intermolecular interactions can be analyzed in the pre-polymerization process using computer simulation, UV-Vis spectroscopy, FTIR, and
1H-NMR. Meanwhile, Suspended-State STD HR/MAS NMR, Raman spectroscopy, SERS, and fluorescence spectroscopy were used after MIP formation
[67][80][81][82][83][84][14,16,83,84,85,86]. Hydrogen bonding interactions are the kind of interaction that focuses on all methods as this interaction strongly contributes to the affinity of molecularly imprinted polymers (MIPs), especially for low molecular weight compounds in organic, aprotic solvents
[85][87].