Bariatric/metabolic surgery involves different techniques leading to different effects on pancreatic cell populations. Currently, sleeve gastrectomy (SG) is one of the most performed techniques. A consequence of this procedure is the drastic removal of the gastric fundus and corpus ghrelin-producing cell population.
- sleeve gastrectomy
- roux-en-Y gastric bypass
- beta-cell
- alpha-cell
- epsilon-cell
- islet
- trans-differentiation
1. Introduction
Bariatric/metabolic surgery has been a powerful tool for the treatment of diabetes mellitus for a long time. Sleeve gastrectomy (SG) and roux-en-Y gastric bypass (RYGB) are two of the most performed ones [1,2][1][2] as Figure1 shows.
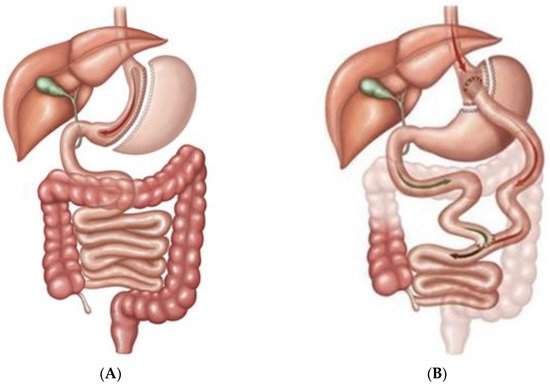
Changes in energy homeostasis and body fat mass have been proposed as a primary mechanism to explain these phenomena [3[3][4],4], but other mechanisms such as changes in several gastrointestinal hormones also seem to be involved with a large number of publications written on the topic. Many of them have related the anatomical changes in the gastrointestinal tract after surgery with the modification of serum levels of glucagon like peptide-1 (GLP-1) [5], ghrelin [6], peptide tyrosine-tyrosine (PYY) [7], gastrointestinal inhibitory peptide (GIP) [8], or even leptin [9], among others, in humans and animal models. Their involvement is clear, but the exact mechanisms and their degree of participation remain partially unknown.
At the other end of the entero-pancreatic axis, the endocrine pancreas containing Langerhans islets determines changes in carbohydrate metabolism after bariatric/metabolic surgery. Their hormonal secretions before and after bariatric/metabolic surgery have been widely studied in plasma or serum from animals and humans [10,11][10][11] but the islet cell composition and its paracrine interactions have been studied less. We will attempt to summarize what we know about the subject by means of a bibliographical review of the most relevant works published on the subject.
2. Effects of RYGB and SG on Endocrine-Cell Populations in the Pancreas
This paper is a narrative literature review text that aims to expose the framework surrounding the effects of RYGB and SG on endocrine-cell populations in the pancreas. We performed a selective search of numerous articles in different databases, books.
The literature of the main scientific databases was reviewed. The search was limited to documents published between 2001 and 2021. These databases were Medline, PubMed, Chochrane and Scopus. In addition, a search was carried out on academic websites, such as Google Scholar, SciELO and Dialnet. The main Boolean operators used were: AND, OR and NOT, and the key words were sleeve gastrectomy; roux-en-Y gastric bypass; beta-cell, alpha-cell; epsilon-cell; islet; trans-differentiation. Due to the large number of studies found, the following criteria were applied to filter the results and work with the most relevant studies.
Inclusion criteria: Original articles, systematic reviews and meta-analyses concerning modifications of the endocrine pancreas after bariatric or metabolic surgery in humans or animal models. Papers published in English in the last 20 years (2001–2021). We prioritised information from systematic reviews and meta-analyses with high scientific evidence.
In the end, a total of 435 articles were found that met the search criteria. Of these, 47 were selected for the preparation of this manuscript. As e number and percentage of citations selected for the manuscript.
3. Sleeve Gastrectomy and Roux-En-Y Gastric Bypass
Bariatric/metabolic surgery involves different techniques leading to different effects on pancreatic cell populations. Currently, sleeve gastrectomy (SG) is one of the most performed techniques. A consequence of this procedure is the drastic removal of the gastric fundus and corpus ghrelin-producing cell population. This situation leads to 35–45% reduction of blood ghrelin levels after gastrectomy in humans [12,13,14][12][13][14]. However, a recent study described the expansion of the pancreatic residual postnatal epsilon-cell population with recovery of plasma ghrelin levels in rats twelve weeks after SG. This expansion takes place at the expense of pancreatic cell progenitors that differentiate into epsilon-cells showing a high expression of lineage markers such as neurogenin-3 (Ngn-3) but not homeodomain protein Nkx2.2 ( Figure 2 ) [15].

This leads us to believe in an adaptive response of the endocrine pancreas to low circulating ghrelin levels and in a possible explanation of the improvement of beta cell function after SG if we take into account the protective role of ghrelin on it [16].
Furthermore, this surgery does not only affect the epsilon-cells in the islets. It is clear that SG preserves the beta-cell function, at least for a while [17,18][17][18]. This could be explained by the increase of GLP-1 receptor expression in beta cells after SG, implying an increase in paracrine sensitivity to GLP-1 [19,20][19][20]. However, there are doubts about this due to a recent study with a modified mouse model involving an inducible knockdown of GLP-1r in beta-cells (GLP1rβ-cell-ko), which showed improved glycemic profiles, to the wild-nature level, after SG [21]. Other researchers have linked the maintenance of beta-cell mass and beta-cell identity markers such as PDX-1 or MafA [22] (Picture 2) to high levels of gastrin after SG, as well as to correction of long-term blood glucose levels in rodents [23].
On the contrary, the plasticity of the pancreatic alpha-cell population under stressful circumstances is well known. Pregnancy or intermittent fasting are capable of enhancing the alpha-cell mass in mice [40,41][24][25]. Some factors related to the functionality of hepatic glucagon receptors (GCgr) have been proposed as brakes and regulators of alpha-cell population expansion in animal models [42][26]. In this sense, RYGB is also able to cause an increase in the alpha-cell population in mice six months after the operation, including a loss of beta identity markers such as PDX-1 and a gain of alpha-cell markers such as ARX in the islets ( Figure 2 ). All of this suggests long-term trans-differentiation of beta-cells into alpha-cells after surgery [25][27].
4. Conclusions
SG and RYGB are a therapeutic option not only for overweight but also for diabetes. The effects of these surgeries on enterohormonal levels have been extensively studied but on another level, further research on endocrine pancreatic cell populations is also needed. Nevertheless, it seems that different pathophysiological mechanisms underlie each of these surgeries, at least in reference to their pancreatic involvement. This is a complicated issue in humans. However, a better understanding of the mechanisms and cellular dynamics governing these populations after these two surgeries would allow us to limit hypoglycemic episodes, the relapse of diabetes over time or even the development of pharmacological alternatives to the use of bariatric/metabolic surgery.
References
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Leccesi, L.; Nanni, G.; Pomp, A.; Castagneto, M.; Ghirlanda, G.; et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med. 2012, 366, 1577–1585.
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric surgery versus intensive medical therapy for diabetes 5-year outcomes. N. Engl. J. Med. 2017, 376, 641–651.
- Heneghan, H.M.; Nissen, S.; Schauer, P.R. Gastrointestinal Surgery for Obesity and Diabetes: Weight Loss and Control of Hyperglycemia. Curr. Atheroscler. Rep. 2012, 14, 579–587.
- Miras, A.D.; le Roux, C.W. Mechanisms underlying weight loss after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 575–584.
- Larraufie, P.; Roberts, G.P.; McGavigan, A.K.; Kay, R.G.; Li, J.; Leiter, A.; Melvin, A.; Biggs, E.K.; Ravn, P.; Davy, K.; et al. Important Role of the GLP-1 Axis for glucose homeostasis after bariatric surgery. Cell. Rep. 2019, 26, 1399–1408.
- Frezza, E.E.; Chiriva-Internati, M.; Wachtel, M.S. Analysis of the results of sleeve gastrectomy for morbid obesity and the role of ghrelin. Surg. Today 2008, 38, 481–483.
- Camacho-Ramírez, A.; Prada-Oliveira, J.A.; Ribelles-García, A.; Almorza-Gomar, D.; Pérez-Arana, G.M. The leading role of peptide tyrosine tyrosine in glycemic control after roux-en-y gastric bypass in rats. Obes. Surg. 2020, 30, 697–706.
- Honka, H.; Koffert, J.; Kauhanen, S.; Kudomi, N.; Hurme, S.; Mari, A.; Lindqvist, A.; Wierup, N.; Parkkola, R.; Groop, L.; et al. Liver blood dynamics after bariatric surgery: The effects of mixed-meal test and incretin infusions. Endocr. Connect. 2018, 7, 888–896.
- Münzberg, H.; Laque, A.; Yu, S.; Rezai-Zadeh, K.; Berthou, H.R. Appetite and body weight regulation after bariatric surgery. Obes. Rev. 2015, 16, 77–90.
- Dimitriadis, G.K.; Randeva, M.S.; Miras, A.D. Potential Hormone Mechanisms of Bariatric Surgery. Curr. Obes. Rep. 2017, 6, 253–265.
- Meek, C.L.; Lewis, H.B.; Reimann, F.; Gribble, F.M.; Park, A.J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 2016, 77, 28–37.
- Ariyasu, H.; Takaya, K.; Tagami, T.; Ogawa, Y.; Hosoda, K.; Akamizu, T.; Suda, M.; Koh, T.; Natsui, K.; Toyooka, S. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4753–4758.
- Kalinowski, P.; Paluszkiewicz, R.; Wróblewski, T.; Remiszewski, P.; Grodzicki, M.; Bartoszewicz, Z.; Krawczyk, M. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus Roux-en-Y gastric bypass-results of a randomized clinical trial. Surg. Obes. Relat. Dis. 2017, 13, 181–188.
- Popovic, V.; Miljic, D.; Pekic, S.; Pesko, P.; Djurovic, M.; Doknic, M.; Damjanovic, S.; Micic, D.; Cvijovic, G.; Glodic, J.; et al. Low plasma ghrelin level in gastrectomized patients is accompanied by enhanced sensitivity to the ghrelin-induced growth hormone release. J. Clin. Endocrinol. Metab. 2005, 90, 2187–2191.
- Camacho-Ramírez, A.; Mayo-Ossorio, M.A.; Pacheco-García, J.M.; Almorza-Gomar, D.; Ribelles-García, A.; Belmonte-Núñez, A.; Prada-Oliveira, J.A.; Pérez-Arana, M. Pancreas is a preeminent source of ghrelin after sleeve gastrectomy in Wistar rats. Histol. Histopathol. 2020, 35, 801–809.
- Baena-Nieto, G.; Lomas-Romero, I.M.; Mateos, R.M.; Leal-Cosme, N.; Perez-Arana, G.; Aguilar-Diosdado, M. Ghrelin mitigates β-cell mass loss during insulitis in an animal model of autoimmune diabetes mellitus, the BioBreeding/Worcester rat. Diabetes Metab. Res. Rev. 2017, 33, e2813.
- Mullally, J.A.; Febres, G.J.; Bessler, M.; Korner, J. Sleeve Gastrectomy and Roux-en-Y gastric bypass achieve similar early improvements in beta-cell function in obese patients with Type 2 Diabetes. Sci. Rep. 2019, 9, 1880.
- Ugi, S.; Morino, K.; Yamaguchi, T.; Yamamoto, H.; Kaida, S.; Miyazawa, I.; Sato, D.; Sekine, O.; Fujita, Y.; Kashiwagi, A.; et al. Preserving beta-cell function is the major determinant of diabetes remission following laparoscopic sleeve gastrectomy in Japanese obese diabetic patients. Endocr. J. 2019, 66, 817–826.
- Garibay, D.; McGavigan, A.K.; Lee, S.A.; Ficorilli, J.V.; Cox, A.L.; Michael, M.D.; Sloop, K.W.; Cummings, B.P. Beta-cell glucagon-like peptide-1 receptor contributes to improved glucose tolerance after vertical sleeve gastrectomy. Endocrinology 2016, 157, 3405–3409.
- Garibay, D.; Lou, J.; Lee, S.A.; Zaborska, K.E.; Weissman, M.H.; Sloma, E.; Donahue, L.; Miller, A.D.; White, A.C.; Michael, A.D.; et al. β Cell GLP-1R signaling alters α cell proglucagon processing after vertical sleeve gastrectomy in mice. Cell Rep. 2018, 23, 967–973.
- Douros, J.D.; Lewis, A.G.; Smith, E.P.; Niu, J.; Capozzi, M.; Wittmann, A.; Campbell, J.; Tong, J.; Wagner, C.; Mahbod, P.; et al. Share enhanced glucose control following vertical sleeve gastrectomy does not require a beta-Cell Glucagon-Like Peptide 1 Receptor. Diabetes 2018, 67, 1504–1511.
- Li, F.; Cao, H.; Sheng, C.; Sun, H.; Song, K.; Qu, S. Upregulated Pdx1 and MafA contribute to β-cell function improvement by sleeve gastrectomy. Obes. Surg. 2016, 26, 904–909.
- Grong, E.; Kulseng, B.; Arbo, I.B.; Nord, C.; Eriksson, M.; Ahlgren, U.; Mårvik, R. Sleeve gastrectomy, but not duodenojejunostomy, preserves total beta-cell mass in Goto-Kakizaki rats evaluated by three-dimensional optical projection tomography. Surg. Endosc. 2016, 30, 532–542.
- Quesada-Candela, C.; Tudurí, E.; Marroquí, L.; Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Morphological and functional adaptations of pancreatic alpha-cells during late pregnancy in the mouse. Metabolism 2020, 102, 153963.
- Marinho, T.S.; Borges, C.C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Intermittent fasting benefits on alpha- and beta-cell arrangement in diet-induced obese mice pancreatic islet. J. Diabetes Complicat. 2019, 26, 107497.
- Longuet, C.; Robledo, A.M.; Dean, E.D.; Dai, C.; Ali, S.; McGuinness, I.; de Chavez, V.; Vuguin, P.M.; Charron, M.J.; Powers, A.C.; et al. Liver-specific disruption of the murine glucagon receptor produces alpha-cell hyperplasia: Evidence for a circulating alpha-cell growth factor. Diabetes 2013, 62, 1196–1205.
- Bancalero-de los Reyes, J.; Camacho-Ramírez, A.; Fernández-Vivero, J.; Ribelles-García, A.; Macías-Rodríguez, M.; Almorza-Gomar, D.; Carrasco-Molinillo, C.; Mayo-Ossorio, M.A.; Prada-Oliveira, J.A.; Perez-Arana, G. Glucagon-producing cell expansion in Wistar rats. Changes to islet architecture after sleeve gastrectomy. Obes. Surg. 2021, 31, 2241–2249.
