Camellia genus (Theaceae) is comprised of world famous ornamental flowering plants. C. japonica L. and C. sasanqua Thunb are the most cultivated species due to their good adaptation. The commercial interest in this plant linked to its seed oil increased in the last few years due to its health attributes, which significantly depend on different aspects such as species and environmental conditions. Therefore, it is essential to develop fast and reliable methods to distinguish between different varieties and ensure the quality of Camellia seed oils. The present work explores the study of Camellia seed oils by species and location. Two standardized gas chromatography methods were applied and compared with that of data obtained from proton nuclear magnetic resonance spectroscopy (1H-NMR) for fatty acids profiling. The principal component analysis indicated that the proposed 1H-NMR methodology can be quickly and reliably applied to separate specific Camellia species, which could be extended to other species in future works.
1. Introduction
Camellia is a genus of flowering plants in the family
Theaceae, native to East Asia and widely distributed in China, India, Japan, and South-East Asian countries, whose seeds and leaves present high nutritional and medicinal values. This subtropical evergreen shrub or small tree arrived in Europe around the 16th century
[1], and was introduced into the gardens of the highest social classes of Galicia (NW of Spain) at the beginning of the 19th.
Nowadays, cultivars of
Camellia species are found worldwide in public and private gardens thanks to their excellent adaptation to climatic and edaphic conditions, easy spread, and resistance to pests and diseases. Particularly,
Camellia japonica L. is the best known internationally as a cultivated species for ornamental value. In the last decade, commercial interest was remarkable, and consequently, production in Spain reached about 2.5 million
Camellia plants per year, which are exported throughout Europe as ornamentals
[2][3][4][2,3,4].
Camellia oil is obtained from the seeds, known as one of the most popular edible vegetable oils that was utilized for more than 1000 years in China, and also abundantly used in southeast Asian countries (Japan, Korea, India, Sri Lanka, Indonesia, and Vietnam), where
Camellias are abundantly available
[5].
Camellia oil is also known as “Eastern Olive Oil” because it shares a similar chemical composition with olive oil
[6]. It contains several natural antioxidants, such as squalene, phytosterol, polyphenols, fat-soluble vitamins (vitamins A, B, E), sasanqua saponin, and other functional substances. It was recommended by the Food and Agriculture Organization of the United Nations as a high-quality, healthy vegetable oil because of its nutritional value and excellent storage qualities
[7]. For these reasons, it is commonly used as cooking oil (edible oil)
[8][9][8,9]. In China, the main species used for oil production is
Camellia oleifera C. Abel
[10], while in Japan this is
C. japonica [11], and
C. sasanqua in Vietnam
[12].
Camellia oil is an expensive product with a particular and characteristic aroma and taste, good storage stability, and high nutritional and medicinal values, with high value interest for trade
[13]. Thus, the economic interest in this crop increased exponentially in recent years for a variety of purposes
[14]. Specifically,
Camellia oil extracted from seeds of different species, including
C. reticulata Lindl.,
C. sinensis L.,
C. oleifera, and
C. japonica, was long processed as an industrial oil used for oligosaccharide production
[15], as a surfactant, in soaps, as a hair oil, and now it is generating interest as a biofuel source, lubricant, and biopolymer
[16][17][18][19][20][16,17,18,19,20]. Although, in cosmetics
C. japonica oil has a long history of traditional cosmetic usage in Japan as a protectant to maintain skin and hair health, where other species are nowadays commonly used for this purpose (e.g.,
C. oleifera, C. grijsii Hance, and
C. sasanqua)
[11][21][11,21].
Camellia oil has fat-soluble natural compounds with health benefits, reducing cholesterol and triglycerides in the blood, lowering blood pressure, and promoting effects such as antioxidation, antipermeability, anti-inflammation, as an analgesic, and anticancer properties
[22][23][24][22,23,24], as well as antimicrobial and antiviral activities
[25]. In addition to this, they are used in traditional treatments in China to prevent cardiovascular diseases, arteriosclerosis, and burn injuries
[26][27][28][26,27,28].
Triacylglycerols are the principal components of
Camellia oils, with a high proportion of oleic and linoleic acids and low saturated acids. This general lipidic profile is associated with well-known health properties. The oil yield of seeds from this plant is high, being on average 30% oil per seed. However, the seed oil content varies according to species, cultivar, and environmental conditions
[29][30][29,30]. The profile of fatty acids (FAs) allows correlation to be made with their botanical origin, which is a very important aspect from a commercial point of view, since the traceability of these oils is mandatory to avoid fraud by adulteration. The properties of the oils are also dependent on the FAs’ composition. The degree of unsaturation and chain length, and the presence of polyunsaturated FAs, appear to increase the potential beneficial properties of these oils
[31]. The unsaturated FAs content in
Camellia oil can reach as much as 90%, which is the highest amount so far reported for unsaturated FAs in edible oils
[22][32][33][22,32,33]. In recent years,
Camellia oil became one of the most popular and expensive edible vegetable oils on the market in China, being more susceptible to adulteration with other cheaper oils by unscrupulous traders for high profits. Another aspect of fraud, the mislabeling of oil extraction methods, and geographical or origin, also destabilize the local
Camellia oil market economies
[34]. The method for
Camellia oil authentication currently used officially, employing gas chromatography (GC) techniques, includes the FAs’ composition. The increased demand for
Camellia oil made the development of rapid and reliable methods for the unequivocal chemical plant species oil characterization associated with the quality of the edible oil a priority objective to avoid commercialization of adulterated
Camellia oils
[35][36][37][38][39][35,36,37,38,39].
To determine the FA composition, a wide variety of analytical methods are available. In this context, traditional methods are gas chromatography with flame ionization detectors (GC-FID)
[40] or gas chromatography-mass spectrometry (GC-MS)
[41]. In these methods, a pretreatment of the sample is necessary to convert the FA into the corresponding methyl esters (FAMEs). So, these methodologies are tedious, time-consuming, require the use of FAs standards, and involve complicated pretreatment of the samples prior to analysis, such as the triacylglycerol hydrolysis and esterification that could face problems of oxidation during the derivatization process
[42][43][44][42,43,44].
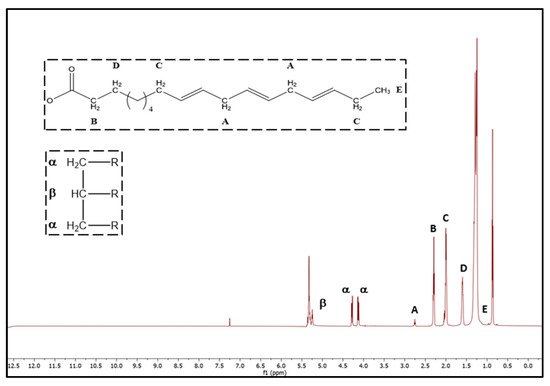
Currently, new, rapid, and nondestructive methods such as Near-InfraRed (NIR), Raman Spectroscopy, and Nuclear Magnetic Resonance (NMR) techniques were recognized as alternative analytical tools in combination with appropriate chemometrics in oil quality control
[45]. Specifically, recent studies confirmed that NMR is a powerful tool for qualitative and quantitative analysis of FAs composition in edible vegetable oils
[32][40][46][47][48][49][50][32,40,46,47,48,49,50].
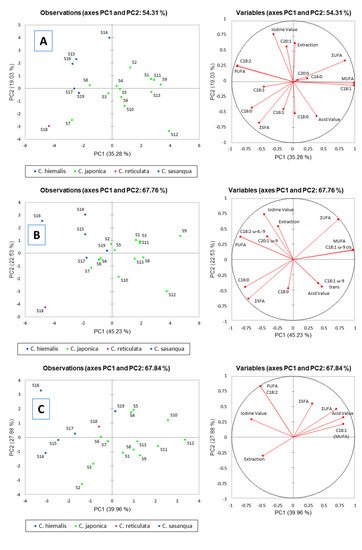
2. Results and Discussion on Camellia Seed Oils
2.1. Oil Content
Seeds of all
Camellia species contain oil. However, oil content and quality may vary with species
[51]. High seed oil variability is likely the result of several factors, including environmental variables such as soil, altitude, light, rainfall, humidity, and temperature, all playing a key role, as previously demonstrated for a variety of plants
[30]. Thus, seed oil content (SOC) of traditional
Camellia varieties can range between 24% and 50%, with an average about 30%
[29].
C. oleifera, which is the earliest species exploited for edible oil, accounting for 98% of the
Camellia cultivated area in China, was previously reported to provide an SOC between 21% and 34%
[52]. Moreover, some of the new
C. oleifera cultivars can reach as much as 53% oil per dry seed
[53].
In this study, seeds from different
Camellia species (
C. japonica,
C. sasanqua,
C. reticulata, and
C. hiemalis Nakai) were harvested in various locations in the province of Pontevedra (Galicia, NW Spain,
Figure 1) during the last four months of 2019. The percentage of seed oil extracted from
Camellias varied from 16.1% to 31.9% for
C. japonica, and from 22% to 30.1% for
C. sasanqua, providing mean values of 23.1% and 25.8%, respectively (
Table 1). Thus, both species are appropriate candidates for use in
Camellia oil production.
C. reticulata and
C. hiemalis showed slightly lower values of 16.6% and 22.6%, respectively.
Figure 1. Camellia locations.
Table 1. Origin and quality parameters of Camellia seed oils.
| Sample |
Species |
Origin-Code |
Harvest |
Extraction Yield |
Acid Value |
Iodine Value |
| C20:1 ω-9 |
∑SFA |
MUFA |
PUFA |
∑UFA |
(w/w, %) |
(mg KOH/g Oil) |
(g I2/100 g Oil) |
|---|
| 1 |
C. japonica |
Cuntis |
Sep. |
26.0 |
5.61 ± 0.02 jk |
79.1 ± 0.5 de |
| 1 |
6.69 ± 0.00 c |
1.66 ± 0.02 ef |
87.1 ± 0.1 gh |
0.72 ± 0.03 d–f |
3.59 ± 0.02 d |
0.25 ± 0.01 ab |
8.35 |
88.07 |
3.59 |
91.65 |
| 2 |
C. japonica |
EFA-826 |
Sep. |
31.9 |
0.39 ± 0.00 b |
82.2 ± 0.0 g |
| 2 |
7.44 ± 0.07 fg |
1.94 ± 0.04 ij |
86.5 ± 0.1 fg |
0.76 ± 0.04 d–g |
3.08 ± 0.03 bc |
0.24 ± 0.02 bc |
9.38 |
87.53 |
3.08 |
90.62 |
3 |
C. japonica |
EFA-942 |
Sep. |
21.6 |
1.81 ± 0.02 e |
82.2 ± 0.2 g |
| 3 |
6.84 ± 0.03 cd |
1.64 ± 0.02 d–f |
87.7 ± 0.1 hi |
0.66 ± 0.03 c–e |
3.12 ± 0.05 bc |
ND |
8.48 |
88.43 |
3.12 |
91.52 |
4 |
C. japonica |
Quiñones de León/O Castro-876 |
Aug. |
24.0 |
5.55 ± 0.04 j |
83.2 ± 0.1 gh |
| 4 |
7.80 ± 0.06 hi |
1.68 ± 0.01 e–g |
85.3 ± 0.2 c–e |
0.92 ± 0.06 h |
4.06 ± 0.09 ef |
0.22 ± 0.00 a |
9.48 |
86.43 |
4.06 |
90.52 |
5 |
C. japonica |
Quiñones de León/O Castro-877 |
Aug. |
24.0 |
5.66 ± 0.00 k |
85.6 ± 0.0 i |
| 5 |
7.51 ± 0.03 gh |
1.58 ± 0.01 de |
86.1 ± 0.1 ef |
0.90 ± 0.07 gh |
3.75 ± 0.04 de |
0.20 ± 0.01 a |
9.09 |
87.17 |
3.75 |
90.91 |
6 |
C. japonica |
Pazo de Lourizán |
Sep. |
28.4 |
5.60 ± 0.01 jk |
78.7 ± 0.4 cd |
| 6 |
8.04 ± 0.17 i |
1.49 ± 0.02 cd |
85.5 ± 0.3 de |
0.74 ± 0.01 d–f |
4.27 ± 0.09 f–h |
ND |
9.53 |
86.20 |
4.27 |
90.47 |
7 |
C. japonica |
Pazo de Gandarón |
Aug. |
23.2 |
4.52 ± 0.04 i |
76.5 ± 0.1 b |
| 7 |
8.46 ± 0.01 j |
0.89 ± 0.02 a |
84.6 ± 0.1 c |
0.89 ± 0.02 gh |
4.35 ± 0.06 f–h |
ND |
9.35 |
85.53 |
4.35 |
89.86 |
8 |
C. japonica |
Castelo de Soutomaior |
Sep. |
19.7 |
5.61 ± 0.00 jk |
80.9 ± 0.2 f |
| 8 |
6.92 ± 0.19 c–e |
1.86 ± 0.03 g–i |
87.6 ± 0.4 hi |
0.66 ± 0.03 c–e |
3.01 ± 0.14 b |
ND |
8.78 |
88.23 |
3.01 |
91.22 |
| 9 |
6.07 ± 0.02 a |
1.27 ± 0.01 b |
89.2 ± 0.1 j |
0.62 ± 0.04 c–e |
2.79 ± 0.06 b |
ND |
7.34 |
89.87 |
2.79 |
92.66 |
| 10 |
7.45 ± 0.11 fg |
2.05 ± 0.08 j |
85.5 ± 0.6 de |
1.18 ± 0.06 i |
3.49 ± 0.39 cd |
| 3.02 |
| 90.92 |
| 14 |
7.11 ± 0.10 de |
1.78 ± 0.03 f–h |
85.6 ± 0.2 de |
0.53 ± 0.01 a–c |
4.54 ± 0.05 gh |
0.41 ± 0.00 d |
8.89 |
86.57 |
4.54 |
91.11 |
| 15 |
7.17 ± 0.03 e |
1.97 ± 0.01 j |
85.2 ± 0.1 cd |
0.57 ± 0.00 b–d |
4.70 ± 0.03 h |
0.38 ± 0.00 d |
9.14 |
86.13 |
4.70 |
90.86 |
| 16 |
7.50 ± 0.11 gh |
1.84 ± 0.04 g–i |
83.4 ± 0.3 b |
0.42 ± 0.04 a |
6.53 ± 0.15 i |
0.33 ± 0.01 c |
9.34 |
84.13 |
6.53 |
90.66 |
| 17 |
8.39 ± 0.06 j |
1.46 ± 0.05 bc |
85.2 ± 0.3 cd |
0.75 ± 0.05 d–h |
4.21 ± 0.20 fg |
ND |
9.85 |
85.93 |
4.21 |
90.15 |
| 18 |
9.32 ± 0.07 k |
2.64 ± 0.02 kl |
83.3 ± 0.2 b |
0.46 ± 0.02 ab |
4.03 ± 0.15 ef |
0.21 ± 0.01 a |
11.96 |
84.00 |
4.03 |
88.04 |
| 19 |
7.84 ± 0.07 i |
1.28 ± 0.03 b |
86.0 ± 0.1 ef |
0.65 ± 0.04 c–f |
4.23 ± 0.03 fg |
ND |
9.12 |
86.67 |
4.23 |
90.88 |