Sulfated fucose-rich polysaccharides from marine organisms are unique molecules with various pharmacological effects. They might have promising therapeutic applications in different diseases.
- sulfated fucose-rich polysaccharides
- sulfated fucan
- fucosylated chondroitin sulfate
- fucoidan
- oral administration
- anticoagulant activity
- marine organisms
1. Introduction
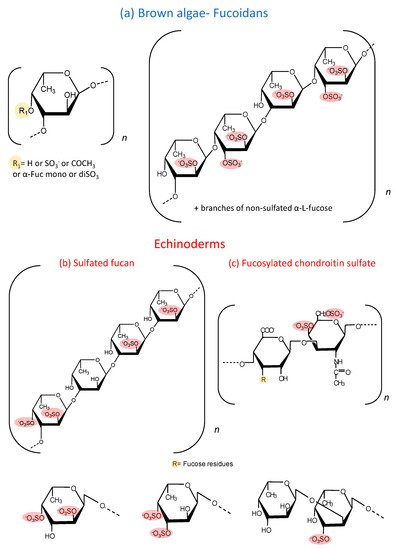
Moreover, sea cucumbers possess another polysaccharide rich in sulfated fucose, denominated as fucosylated chondroitin sulfate (fucCS) [1]. This compound has a central chain similar to chondroitin sulfate from vertebrates, but it has branches of fucose linked to position 3 of glucuronic acid of the central core. The structure of these fucose branches varies between species. Figure 1 c shows the structure of this sulfated polysaccharide. Table 1 and Table 2 show the structural characteristics of fucoidans and fucCS cited in this manuscript. It is important to emphasize that fucoidans can show structural variation according to the source, extraction method and time of the year [2].
| Species | Structure | Sugar and Sulfate Content | Mw (kDa) | Ref | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. nodosum | 1→3)-α-l-Fucp and a few (1→4)-α-l-Fucp with →3-α-l-(2 and/or 4 Fucp) | The carbohydrate and sulfate content of the fraction A3 were 74.7% and 12.0%, respectively. | 97.52 | [12][3] | |||||||
| S. henslowianum | →3)-α-l-Fucp(2 SO3−)-(1→3)-α-l | ||||||||||
| I. badionotus | -Fucp (4 SO3−)-(1→ | Fucose and glucose as main sugars. Sulfate content: 25.20%. | ND | 4.1% α-Fuc-4SO4, 95.9% α-Fuc-2,4diSO[4] | |||||||
| 4 | 70.4 kDa | [ | 13] | F. evanescens | →3)-α-l-Fucp(2 SO3−)-(1→4)-α-l-Fucp(2 SO3−)-(1→ | Fucose, sulfate and acetyl groups at a molar ratio of 1:1.23:0.36 and trace amounts of galactose and xylose. | 10–100 | [5] | |||
| L. grisea | ~27% α-Fuc-2,4diSO4; ~20% α-Fuc3,4diSO4 and ~53% disaccharides composed of α-Fuc1→2-α-Fuc-3SO4→ | 40 kDa | [14 | F. vesiculosus | →3)-α-l-Fucp(2 SO3−)-(1→4)-α-l-Fucp(2,3-SO3−)-(1→3) | 55.9% of carbohydrates, 27.0% of sulfate residues and 5.7% of uronic acid. Carbohydrates were represented mainly by fucose (38%), galactose (3.5%), xylose (2.7%). | 20.7 | [6] | |||
| ] | |||||||||||
| C. frondosa | The chemical composition contained mainly glucuronic acid, galactosamine and fucose in the molar ratio of 1:1.50:1.16, with 30.07% sulfate content. | 14.76 kDa | [15] | C. okamuranus | →3)-α-l-Fucp (SO3−)-(1→3)-α-l-Fucp(4 SO3−)-(1→ | The glucuronic acid residues are linked to the C-2 positions of the fucose residues, which are not substituted by a sulfate group. Sulfate content ~15%. | 92.1 | )-(1→3) | 79.49% of fucose and 16.76% of galactose. Sulfate content ~30.72%. | 30 | [9] |
| 300 | [ | 11 | ] |
| Species | Proportions of the Branching Sulfated Fucose Units | Mw (kDa) | Ref | |
|---|---|---|---|---|
| P. graeffei | 81.6% α-Fuc-4SO4, 18.4% α-Fuc-2,4diSO4 | 49 kDa | ||
| [ | ||||
| 7 | ||||
| ] | ||||
| U. pinnatifida | →3)-α-l-Fucp(2 SO3−)-(1→4)-α-l-Fucp(2,3-di SO3−)-(1→3) | This sulphated galactofucan is composed of: galactose 44.6% and fucose 50.9%. Xylose (4.2%), mannose (0.3%). Sulfate content 15%. A significant number of O-acetyl groups. | 378 | [8] |
| S. japonica | →3)-α-l-Fucp(2 SO3−)-(1→4)-α-l-Fucp(2,3-di SO3− | |||
| Mozuku (High molecular weight fraction) | ND | Sulfate content: 13%. | 240 | [10] |
| L. japonica | →3)-α-l-Fucp(4 SO3−)-(1→ | 46.5% fucoxanthin, 8.01% lipids and 45.4% carbohydrates of mostly cellulose. Sulfate content: 13%. |
These sulfated polysaccharides from echinoderms allowed a significant advance in the attempts to correlate structure and biological activities of these molecules, which is difficult to establish with fucoidans [16]. Many studies report the biological effects of the fucose-rich sulfated polysaccharides administered intravascularly, subcutaneously or intraperitoneally [17][18][19][20][21]. More recently, studies have emerged reporting several pharmacological effects of these polysaccharides after oral administration [14][22]. The observation that these compounds have a therapeutic effect after their oral administration is very significant since it opens the perspective of the use of these molecules for the development of new drugs. The oral route is more practical, comfortable and preferred by patients for long term treatments.
The purpose of this review was to conduct a systematic analysis of the effects observed after oral administration of the sulfated fucose-rich polysaccharides. Researchers distinguished the effects observed with the complex fucoidans from algae from those obtained with the echinoderm polysaccharides. The use of fucCS and SFs is an important pharmacological tool to define structure versus therapeutic effects of polysaccharides rich in fucose as a basis for the development of new drugs.
Structural characteristics of fucoidans with pharmacological activities after oral administration.
2. Anticancer Effect
Cancer is a leading cause of death, along with cardiovascular diseases. The hallmark of cancer treatment has been conventional chemotherapy. Chemotherapeutic drugs target rapidly dividing cells, such as cancer cells; however, these drugs also target normal cells, such as intestinal epithelium, bone marrow and hair follicles. In an attempt to target only cancer cells, a new generation of anticancer drugs arises using specific monoclonal antibodies, small molecule inhibitors and immunotoxins [23]. However, side effects and emerging resistance are still an issue, which increase the demand for new compounds that could act as adjuvant therapy and/or increase efficacy [24]. Regarding this issue, some data in the literature explore the anticancer effect of sulfated polysaccharides after oral administration. Table 3 summarizes the major observations.
Table 3. Anticancer effects of sulfated fucose-rich polysaccharides after oral administration.
| Polysaccharide | Dosage Regimen and Species | Major Observations and Mechanism Proposed | Ref. |
|---|---|---|---|
| Fucoidan from F. evanescens | 1–50 mg/ kg, 3 times/week/ up to 21 days, Rats | Inhibition of lymphokine-activated killer T-cell-originated protein kinase (TOPK) (64% at 400 µg/mL) and EGF-downstream signaling. ↓ Tumor growth 72% at 50 mg/kg. | [5] |
| Fucoidan from F. vesiculosus | 150 mg/kg/body weight, 2 weeks, Athymic mice | Enhancement of the cytotoxic activity of splenic NK cells (~2.3 fold). | [25] |
| Fucoidan from F. vesiculosus | 100 mg/kg, 21 days starting on the seventh day pos tumor implantation, Mice | Induces G0/G1 cell cycle arrest (2–10%) and caspase-dependent apoptosis. | [26] |
| Fucoidan from F. vesiculosus | 144 mg/kg, 26 days, Mice | Reduction of Transforming Growth Factor Receptor (TGFR) levels (↓ ~50%) and its downstream signaling pathways. Enhancement of TGFR degradation. | [27] |
| Fucoidan from F. vesiculosus | 20 mg/kg, 28 days, Athymic mice | Inhibition of angiogenesis by decreasing mRNA expression level of angiogenesis related markers (↓ ~70%) and gene promoters. | [28] |
An example of the beneficial effect of these polysaccharides as anticancer drugs is the observation that oral doses of fucoidan from Fucus vesiculosus delayed tumor growth in a xenograft model and increased cytolytic activity of natural killer cells [25]. Athymic mice were pre-treated with fucoidan daily for 2 weeks and then a human acute promyelocytic leukemia cell line was injected subcutaneously. Significant antitumor activity was observed without any sign of toxicity. Tumor development was clearly slower in the oral fucoidan-treated mice than in the control group. An enhancement of the cytotoxic activity of splenic natural killer cells in mice that were orally treated with fucoidan was also observed, which could be in part responsible for its pharmacological effect. Interestingly, when fucoidan from the same specie were orally administered for 21 days, starting on the seventh day post-tumor implantation, significant reduction in tumor volume and tumor weight was observed when compared with the control group [26]. In vitro assays showed that fucoidan could induce G 0/G 1 cell cycle arrest and caspase-dependent apoptosis in diffuse large B-cell lymphoma culture. This indicates that oral fucoidan administration can inhibit tumor growth and development.
In another xenograft model using human prostate carcinoma cells, oral administration of fucoidan for 28 days significantly hindered the tumor growth and tumor vascular density, as indicated by hemoglobin quantification assay [28]. The mRNA expression level of CD31 and CD105, biomarkers of endothelium, also declined. Analysis of the protein expression and gene promoters related to angiogenesis showed that their levels were reduced after oral fucoidan treatment, suggesting that fucoidan hindered tumor growth by inhibiting the formation of new blood vessels.
The anticancer effect of fucoidan was also observed using the polysaccharide from brown alga Fucus evanescens using a xenograft model. Colon cancer cells were inoculated into athymic nude mice [5]. Oral treatment with fucoidan for 21 days inhibited tumor growth compared with the vehicle-treated group. The antitumor effect of fucoidan was associated with its inhibition of lymphokine-activated killer T-cell-originated protein kinase (TOPK), highly expressed in many cancers. Tissues from each group were analyzed for phosphorylation of TOPK downstream targets, and the expression of these markers was decreased after 20 days of oral fucoidan treatment. Additional in vitro assays showed that this fucoidan modulates EGF-induced neoplastic transformation of mouse epidermal cells in a concentration-dependent manner. This pathway is related to the machinery that controls fundamental cellular processes, such as growth, proliferation, differentiation, migration and apoptosis. The polysaccharide also binds and decreases TOPK kinase activity in vitro, although a high concentration is required for this effect. The antitumoral activity of the echinoderm polysaccharides has not been tested so far after oral administration. These well-defined structures may help to clarify the effect of sulfated polysaccharides on cancer cells.
3. Immunomodulatory Effect
Immunomodulatory drugs can act at different levels of the immune system. Therefore, different kinds of drugs have been developed that selectively either inhibit or intensify the specific populations of immune responsive cells, i.e., lymphocytes, macrophages, neutrophils, natural killer cells, and cytotoxic T lymphocytes. Immunomodulators affect the cells systems by producing soluble mediators such as cytokines. Therefore, the rational use of drugs with anti-inflammatory effects is necessary to avoid excessive inflammation triggered by external agents or autoimmune diseases and drugs with immunostimulatory effects to increase the immune response such as the production of specific antibodies. In this context, oral administration of sulfated fucose-rich polysaccharides has also shown some interesting effects. A summary of these effects is shown in Table 4 .
| Immunomodulatory Effect | Polysaccharide | Dosage Regimen and Species | Major Observations and Mechanism Proposed | Ref. |
|---|---|---|---|---|
| Antifibrotic effect | Fucoidan from C. okamuranus | Free access to drinking water containing 2% low (28.8 kDa) or high (41.4 kDa) MW fractions, 12 weeks, Rats | ↓ TGF-β1 mRNA expression and the levels of chemokine ligand CXCL12 in the liver (~3 fold). | [7] |
| Hepatoprotection | Fucoidan from F. vesiculosus | 30 or 60 mg/kg, 7 days, Mice | ↓ expression of liver TGF-β1(~40%) and COX-2, ↑ antioxidant pathways. | [29] |
| Hepatoprotection | Fucoidan from F. vesiculosus | 100 mg/kg, 4 weeks, Rats on high-fat diet | ↓TNF-α, IL-1β and MMP-2 mRNA expressions (~50–70%). Prevention of the increase in serum lipids and glucose levels induced by HFD. | [30] |
| Nephroprotection | Fucoidan from F. vesiculosus | 50 and 75 mg/kg, 13 weeks, Rats | Decreased levels of collagen IV, NF-κB, TGF-β1 and fibronectin in the renal cortex and in the glomerular mesangial cells. | [31] |
| Anti-arthritic and antioxidant effects | Fucoidan from U. pinnatifida | 50 or 150 mg/kg, 25 days, Rats | Downregulation of COX-2 and other inflammatory mediators (68% inhibition of in vivo inflammation). | [8] |
| Immunostimulatory effects | Fucoidan from U. pinnatifida | 300 mg daily,20 weeks, Human | Higher immunogenicity of influenza trivalent vaccine than control group and increase of natural killer cell activity. | [32] |
| Suppression of allergic symptoms | Fucoidan from S. japonica | 100–400 μg/day,4 days, Rats | Prevention of the interaction of IgE and mast cells via an increase in galectin-9 mRNA expression (↑ ~50%) in intestinal epithelial cells. | [9] |
| Anti-inflammatory effect | FucCS from I. badionotus | 80 m/kg, 7 days, Rats | Downregulation of NF-kB and downstream genes such as COX-2 and TNF-α and a benefic effect on gut microbiota. | [33] |
| Anti-inflammatory effect | Sulfated fucan from A. molpadioides with varying degrees of polymerization (10–500 kDa) | 50 mg/kg, 26 days, Mice | Regulation of IFN-γ/IL-4 ratio (0.53 to 0.70) and Th1/Th2 response, IL-6 and IL-10 levels, enhanced IgA protein expression levels (~35%) in intestinal mucosa. | [34] |
The impact of the molecular weight on the anti-inflammatory activity of sulfated fucan from the sea cucumber Acaudina molpadioides with varying degrees of polymerization was also reported [34]. The SF was tested on an animal model of intestinal mucositis after oral administration for 26 days. Histological analysis revealed that morphology of the intestinal mucosa of fucoidan-treated animals was similar to the healthy group and that this effect was more pronounced with the high-molecular-weight fractions. Interestingly, these fractions regulated Th1/Th2 immune balance processes by altering IFN-γ/IL-4 ratio, while the oral administration of intact fucoidan had no effect. Intact SF and the high-molecular-weight fractions enhanced IgA protein expression levels in intestinal mucosa and strengthened intestinal adaptive immunity. Another interesting aspect of this work was the analysis of plasma concentration achieved by the oral administration of the SF. The low-molecular-weight fractions achieved high plasma levels when compared with unfractioned polysaccharide; therefore, the absorption and bioavailability of SF are likely to depend on the molecular size of the polysaccharide.
Another approach investigated the anti-inflammatory effect of oral administration of fucCS from the sea cucumber I. badionotus for 7 days. In contrast with the highly heterogeneous fucoidan from brown algae, this polysaccharide has a regular repetitive structure containing mostly 2,4 disulfated fucose units, as shown in Figure 1 c. When tested on an experimental model of colitis induced by dextran sulfate, oral fucCS attenuated the body weight loss, expression of colonic TNF-α gene and colon shortening caused by experimental colitis. The authors proposed that this protective effect might be due to downregulation of NF-kB and downstream genes such as COX-2 and TNF-α and a benefic profile on gut microbiota [33].
Immunomodulatory effects of sulfated fucose-rich polysaccharides after oral administration.
4. Future Perspectives: Pharmacokinetics Studies and Prebiotic Effects
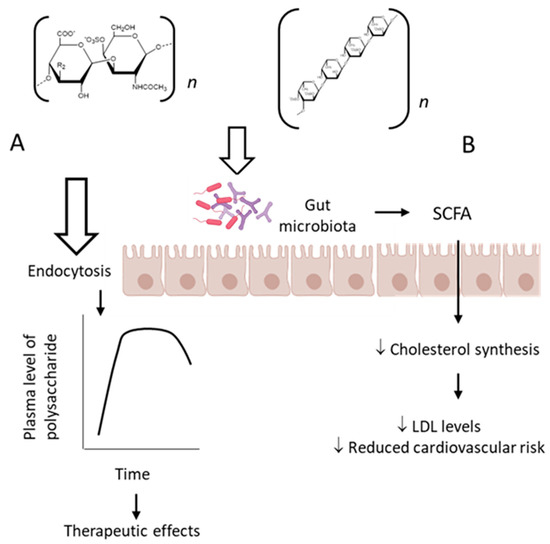
This review summarizes the therapeutic effects achieved after oral administration of sulfated polysaccharides in a variety of pathological processes. These observations were obtained mainly using animal experimental models, although some preliminary data have already been reported in humans. These results are not limited to the therapeutic effect but also highlight the proposed molecular mechanisms involved in the pharmacological action of these polysaccharides. Further studies are necessary to further understand their pharmacokinetic and the modulating effect on the intestinal microbiota.
In the case of heparin, a paradigm of an anticoagulant drug with carbohydrate structure, the transition from intravenous to subcutaneous administration was associated with the development of low-molecular-weight heparin. This led to the development of new analytical methods to study its pharmacodynamics, resulting in the now widespread methods to determine the plasma concentration of heparins based on anti-FXa and anti-FIIa assays [35]. Likewise, there is a need to develop sensitive methods for the study of the pharmacokinetics/pharmacodynamics of fucose-rich polysaccharides after their oral administration.
A partially depolymerized fucCS, administered as a single oral dose of 50 mg/kg, was detected in plasma between 0.5 and 7.5 h using a chromatographic method. Only 0.1% of the dose was detected in the urine accumulated during 24 h [36]. It is very challenging to measure the plasma levels of these polysaccharides analytically because of the heterogeneous molecular weight, branched structure and similarity in monosaccharide composition to mammalian polysaccharides.
The classic mechanism of action proposed for the sulfated fucose-rich polysaccharides after oral administration is summarized in Figure 2 A. The polysaccharides are absorbed through the gastrointestinal tract, probably by endocytosis due to their high molecular weights [37], reach appropriate plasma concentration and exert their therapeutic action. Subsequently, the polysaccharides are distributed to different tissues, metabolized and excreted. Structural modifications might occur during these processes.
References
- Pacheco, R.G.; Vicente, C.P.; Zancan, P.; Mourão, P.A.S. Different Antithrombotic Mechanisms among Glycosaminoglycans Revealed with a New Fucosylated Chondroitin Sulfate from an Echinoderm. Blood Coagul. Fibrinolysis 2000, 11, 563–573.
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–233.
- Yang, Z.; Yin, J.; Wang, Y.; Wang, J.; Xia, B.; Li, T.; Yang, X.; Hu, S.; Ji, C.; Guo, S. The Fucoidan A3 from the Seaweed Ascophyllum Nodosum Enhances RCT-Related Genes Expression in Hyperlipidemic C57BL/6J Mice. Int. J. Biol. Macromol. 2019, 134, 759–769.
- Cuong, H.D.; Thuy, T.T.T.; Huong, T.T.; Ly, B.M.; Van, T.T.T. Structure and Hypolipidaemic Activity of Fucoidan Extracted from Brown Seaweed Sargassum Henslowianum. Nat. Prod. Res. 2015, 29, 411–415.
- Vishchuk, O.S.; Sun, H.; Wang, Z.; Ermakova, S.P.; Xiao, J.J.; Lu, T.; Xue, P.P.; Zvyagintseva, T.N.; Xiong, H.; Shao, C.; et al. PDZ-Binding Kinase/T-LAK Cell-Originated Protein Kinase Is a Target of the Fucoidan from Brown Alga Fucus Evanescens in the Prevention of EGF-Induced Neoplastic Cell Transformation and Colon Cancer Growth. Oncotarget 2016, 7, 18763–18773.
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus Vesiculosus L. Of the Barents Sea. Mar. Drugs 2020, 18, 275.
- Nakazato, K.; Takada, H.; Iha, M.; Nagamine, T. Attenuation of N-Nitrosodiethylamine-Induced Liver Fibrosis by High-Molecular-Weight Fucoidan Derived from Cladosiphon Okamuranus. J. Gastroenterol. Hepatol. 2010, 25, 1692–1701.
- Phull, A.R.; Majid, M.; Haq, I.U.; Khan, M.R.; Kim, S.J. In Vitro and in Vivo Evaluation of Anti-Arthritic, Antioxidant Efficacy of Fucoidan from Undaria Pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480.
- Tanino, Y.; Hashimoto, T.; Ojima, T.; Mizuno, M. F-Fucoidan from Saccharina Japonica Is a Novel Inducer of Galectin-9 and Exhibits Anti-Allergic Activity. J. Clin. Biochem. Nutr. 2016, 59, 25–30.
- Sakai, C.; Abe, S.; Kouzuki, M.; Shimohiro, H.; Ota, Y.; Sakinada, H.; Takeuchi, T.; Okura, T.; Kasagi, T.; Hanaki, K. A Randomized Placebo-Controlled Trial of an Oral Preparation of High Molecular Weight Fucoidan in Patients with Type 2 Diabetes with Evaluation of Taste Sensitivity. Yonago Acta Med. 2019, 62, 14–23.
- Ren, R.; Azuma, Y.; Ojima, T.; Hashimoto, T.; Mizuno, M.; Nishitani, Y.; Yoshida, M.; Azuma, T.; Kanazawa, K. Modulation of Platelet Aggregation-Related Eicosanoid Production by Dietary F-Fucoidan from Brown Alga Laminaria Japonica in Human Subjects. Br. J. Nutr. 2013, 110, 880–890.
- Wei, C.Y.; Liao, N.B.; Zhang, Y.; Ye, X.Q.; Li, S.; Hu, Y.Q.; Liu, D.H.; Linhardt, R.J.; Wang, X.; Chen, S.G. In Vitro Fermentation Behaviors of Fucosylated Chondroitin Sulfate from Pearsonothuria Graeffei by Human Gut Microflora. Int. J. Biol. Macromol. 2017, 102, 1195–1201.
- Zhu, Q.; Lin, L.; Zhao, M. Sulfated Fucan/Fucosylated Chondroitin Sulfate-Dominated Polysaccharide Fraction from Low-Edible-Value Sea Cucumber Ameliorates Type 2 Diabetes in Rats: New Prospects for Sea Cucumber Polysaccharide Based-Hypoglycemic Functional Food. Int. J. Biol. Macromol. 2020, 159, 34–45.
- Fonseca, R.J.C.; Mourão, P.A.S. Fucosylated Chondroitin Sulfate as a New Oral Antithrombotic Agent. Thromb. Haemost. 2006, 96, 822–829.
- Li, S.; Jiang, W.; Hu, S.; Song, W.; Ji, L.; Wang, Y.; Cai, L. Fucosylated Chondroitin Sulphate from Cusumaria Frondosa Mitigates Hepatic Endoplasmic Reticulum Stress and Inflammation in Insulin Resistant Mice. Food Funct. 2015, 6, 1547–1556.
- Pereira, M.S.; Melo, F.R.; Mourão, P.A.S. Is There a Correlation between Structure and Anticoagulant Action of Sulfated Galactans and Sulfated Fucans? Glycobiology 2002, 12, 573–580.
- Mourão, P.A.S. Perspective on the Use of Sulfated Polysaccharides from Marine Organisms as a Source of New Antithrombotic Drugs. Mar. Drugs 2015, 13, 2770–2784.
- Pomin, V.H. Holothurian Fucosylated Chondroitin Sulfate. Mar. Drugs 2014, 12, 232–254.
- Borsig, L.; Wang, L.; Cavalcante, M.C.M.; Cardilo-Reis, L.; Ferreira, P.L.; Mourão, P.A.S.; Esko, J.D.; Pavão, M.S.G. Selectin Blocking Activity of a Fucosylated Chondroitin Sulfate Glycosaminoglycan from Sea Cucumber: Effect on Tumor Metastasis and Neutrophil Recruitment. J. Biol. Chem. 2007, 282, 14984–14991.
- Lee, Y.E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.Y.; Hong, S.C.; Kim, J.T.; Lee, J. Marine Polysaccharides: Therapeutic Efficacy and Biomedical Applications. Arch. Pharmacal Res. 2017, 40, 1006–1020.
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183.
- Richards, C.; Williams, N.A.; Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S.; Park, A.Y. Oral Fucoidan Attenuates Lung Pathology and Clinical Signs in a Severe Influenza a Mouse Model. Mar. Drugs 2020, 18, 246.
- Kumar, M.; Nagpal, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Singh, S.; Marotta, F.; Jain, S.; Yadav, H. Targeted Cancer Therapies: The Future of Cancer Treatment. Acta Biomed. 2012, 83, 220–233.
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural Compounds and Combination Therapy in Colorectal Cancer Treatment. Eur. J. Med. Chem. 2018, 144, 582–594.
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Karpiniec, S.S.; Dickinson, J.L. Fucoidan Suppresses the Growth of Human Acute Promyelocytic Leukemia Cells In Vitro and In Vivo. J. Cell. Physiol. 2016, 231, 688–697.
- Yang, G.; Zhang, Q.; Kong, Y.; Xie, B.; Gao, M.; Tao, Y.; Xu, H.; Zhan, F.; Dai, B.; Shi, J.; et al. Antitumor Activity of Fucoidan against Diffuse Large B Cell Lymphoma in Vitro and in Vivo. Acta Biochim. Sinica 2015, 47, 925–931.
- Hsu, H.Y.; Lin, T.Y.; Wu, Y.C.; Tsao, S.M.; Hwang, P.A.; Shih, Y.W.; Hsu, J. Fucoidan Inhibition of Lung Cancer in Vivo and in Vitro: Role of the Smurf2-Dependent Ubiquitin Proteasome Pathway in TGFβ Receptor Degradation. Oncotarget 2014, 5, 7870–7885.
- Rui, X.; Pan, H.F.; Shao, S.L.; Xu, X.M. Anti-Tumor and Anti-Angiogenic Effects of Fucoidan on Prostate Cancer: Possible JAK-STAT3 Pathway. BMC Complement. Altern. Med. 2017, 17, 1–8.
- Lim, J.D.; Lee, S.R.; Kim, T.; Jang, S.A.; Kang, S.C.; Koo, H.J.; Sohn, E.; Bak, J.P.; Namkoong, S.; Kim, H.K.; et al. Fucoidan from Fucus Vesiculosus Protects against Alcohol-Induced Liver Damage by Modulating Inflammatory Mediators in Mice and Hepg2 Cells. Mar. Drugs 2015, 13, 1051–1067.
- Heeba, G.H.; Morsy, M.A. Fucoidan Ameliorates Steatohepatitis and Insulin Resistance by Suppressing Oxidative Stress and Inflammatory Cytokines in Experimental Non-Alcoholic Fatty Liver Disease. Environ. Toxicol. Pharmacol. 2015, 40, 907–914.
- Wang, Y.; Nie, M.; Lu, Y.; Wang, R.; Li, J.; Yang, B.; Xia, M.; Zhang, H.; Li, X. Fucoidan Exerts Protective Effects against Diabetic Nephropathy Related to Spontaneous Diabetes through the NF-ΚB Signaling Pathway in Vivo and in Vitro. Int. J. Mol. Med. 2015, 35, 1067–1073.
- Negishi, H.; Mori, M.; Mori, H.; Yamori, Y. Supplementation of Elderly Japanese Men and Women with Fucoidan from Seaweed Increases Immune Responses to Seasonal Influenza Vaccination. J. Nutr. 2013, 143, 1794–1798.
- Olivera-Castillo, L.; Grant, G.; Kantún-Moreno, N.; Barrera-Pérez, H.A.; Montero, J.; Olvera-Novoa, M.A.; Carrillo-Cocom, L.M.; Acevedo, J.J.; Puerto-Castillo, C.; Solís, V.M.; et al. A Glycosaminoglycan-Rich Fraction from Sea Cucumber Isostichopus Badionotus Has Potent Anti-Inflammatory Properties in Vitro and in Vivo. Nutrients 2020, 12, 1698.
- Zuo, T.; Li, X.; Chang, Y.; Duan, G.; Yu, L.; Zheng, R.; Xue, C.; Tang, Q. Dietary Fucoidan of Acaudina Molpadioides and Its Enzymatically Degraded Fragments Could Prevent Intestinal Mucositis Induced by Chemotherapy in Mice. Food Funct. 2015, 6, 415–422.
- Mulloy, B.; Hogwood, J.; Gray, E.; Lever, R.; Page, C.P. Pharmacology of Heparin and Related Drugs. Pharmacol. Rev. 2015, 68, 76–141.
- Imanari, T.; Washio, Y.; Huang, Y.; Toyoda, H.; Suzuki, A.; Toida, T. Oral Absorption and Clearance of Partially Depolymerized Fucosyl Chondroitin Sulfate from Sea Cucumber. Thromb. Res. 1999, 93, 129–135.
- Conte, A.; Volpi, N.; Palmieri, L.; Bahous, I.; Ronca, G. Biochemical and Pharmacokinetic Aspects of Oral Treatment with Chondroitin Sulfate. Arzneim. Forsch. Drug Res. 1995, 45, 918–925.
