Panax quinquefolium L. (American Ginseng, AG) is an herb characteristic for regions of North America and Asia. Nowadays, it is one of the most commonly applied medical herbs worldwide. Active compounds of AG are ginsenosides, saponins of the glycosides group that are abundant in roots, leaves, stem, and fruits of the plant. Ginsenosides are suggested to be primarily responsible for health-beneficial effects of AG. AG acts on the nervous system; it was reported to improve the cognitive function in a mouse model of Alzheimer’s disease, display anxiolytic activity, and neuroprotective effects against neuronal damage resulting from ischemic stroke in animals, demonstrate anxiolytic activity, and induce neuroprotective effects against neuronal damage in ischemic stroke in animals.
- Panax quinquefolium L.
- ginsenosides
- anti-cancer activity
- anti-diabetic potential
- antimicrobial effect
1. Introduction
2. American Ginseng: Cultivation, Characteristics, and Applications
3. Bioactive Phytochemicals of American Ginseng
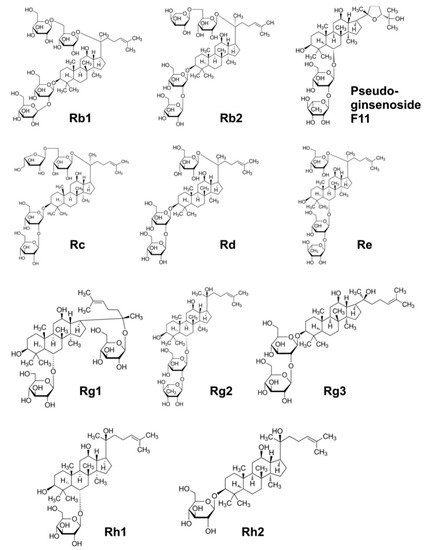
|
Pharmacological Action |
Ginsenoside |
Reference |
|---|---|---|
|
Positively affects memory processes; induces synthesis of acetylcholine in the hippocampus by stimulating choline acetyltransferase; induces apoptosis and inhibits angiogenesis in cancer cells; inhibits the release of inflammatory leukotrienes; reversibly and tonically blocks voltage-dependent Na+ channels in the brain reducing detrimental effects of hypoxia; downregulates the COX-2 gene; stabilises neutrophils and lymphocytes; inhibits the release of histamine; blocks calcium channels and stabilised the heart; reduces blood sugar levels; anti-diabetic, insulin-sensitising and anti-obesity actions; neurotropic, neuroprotective, oestrogen-like activity; stimulates GABA receptors and induces a depressive effect on brain function, which underlines its calming, anxiolytic, sleeping, relaxing and antipsychotic effects |
Rb1, Rb2, Rc |
|
|
Stimulates superoxide dismutase; inhibits angiogenesis in cancer; prevents diabetes; lowers cholesterol and triglycerides levels, activates lipolysis; corticotropic and oestrogenic activity |
Rb2 |
|
|
Inhibits proliferation of breast cancer cells; induces corticotropic effects |
Rc |
|
|
Promotes neurites outgrowth, an important process for neuronal repair; induces corticotropic effects |
Rd, Rc, Re |
|
|
Scavenges hydroxyl radicals and degrades H2O2; reduces blood sugar levels; induces cardioprotective effects; activates cGMP and relaxes smooth muscles |
Re |
|
|
Downregulates the COX-2 gene; stabilises neutrophils and lymphocytes; inhibits histamine release; inhibits platelet-induced activation of thromboxane; increases insulin receptors; increases T-helper lymphocytes; inhibits release of endothelin and relaxation of the smooth muscle of blood vessels; activates cyclic guanosine monophosphate and relaxes the smooth muscle (hypotensive effect); blocks calcium channels and stabilised the heart; reduces blood sugar levels |
Rg1 |
|
|
Inhibits neuronal acetylcholine |
Rg2 |
[22] |
|
Inhibits platelet aggregation induced by thrombin; relaxes the smooth muscle of the blood vessels by activating the K+ channels and releases Ca2+; inhibits progression of tumours and reduces drug resistance of cancer cells; inhibits endothelin and relaxation of the smooth muscle of blood vessels; induces hypotensive effect; downregulates the COX-2 gene; stabilises neutrophils and lymphocytes; inhibits histamine release; modulates mitogen-activated protein kinases, thus inhibiting the spread of cancer cells |
Rg3 |
|
|
Activates oestrogen receptor; inhibits proliferation of cancer cells and induces apoptosis |
Rh1 |
|
|
Inhibits breast, liver and prostate cancer cells proliferation |
Rh2 |
|
|
Assists memory improvement; induces neuroprotective effects |
Pseudoginse-noside F11 |
[22] |
4. Pro-Health Effects of American Ginseng
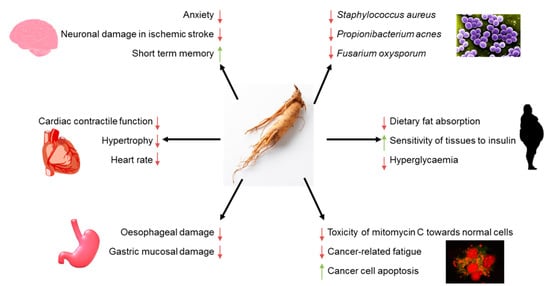
5. Interactions of American Ginseng with Microorganisms
5.1. Antimicrobial Action
5.2. Metabolism of American Ginseng Ginsenosides by Intestinal Microbiota
References
- Ahmad, Z.; Hassan, S.S.; Azim, S. A therapeutic connection between dietary phytochemicals and ATP synthase. Curr. Med. Chem. 2017, 24, 3894–3906.
- Lee, G.; Bae, H. Therapeutic effects of phytochemicals and medicinal herbs on depression. BioMed Res. Int. 2017, 2017, 6596241.
- He, Y.S.; Sun, W.; Wang, C.Z.; Qi, L.W.; Yang, J.; Li, P.; Wen, X.D.; Yuan, C.S. Effects of American ginseng on pharmacokinetics of 5-fluorouracil in rats. Biomed. Chromatogr. 2014, 29, 762–767.
- Li, J.; Ichikawa, T.; Jin, Y.; Hofseth, L.J.; Nagarkatti, P.; Nagarkatti, M.; Windust, A.; Cui, T. An essential role of Nrf2 in American ginseng-mediated anti-oxidative actions in cardiomyocytes. J. Ethnopharmacol. 2010, 130, 222–230.
- Shergis, J.L.; Di, Y.M.; Zhang, A.L.; Vlahos, R.; Helliwell, R.; Ye, J.M.; Xue, C.C. Therapeutic potential of Panax ginseng and ginsenosides in the treatment of chronic obstructive pulmonary disease. Complement. Ther. Med. 2014, 22, 944–953.
- Rotem, C.; Kaplan, B. Phyto-female complex for the relief of hot flushes, night sweats and quality of sleep: Randomized, controlled, double-blind pilot study. Gynecol. Endocrinol. 2007, 23, 117–122.
- McGraw, J.B.; Lubbers, A.E.; Van der Voort, M.; Mooney, E.H.; Furedi, M.A.; Souther, S.; Turner, J.B.; Chandler, J. Ecology and conservation of ginseng (Panax quinquefolius) in a changing world. Ann. N. Y. Acad. Sci. 2013, 1286, 62–91.
- Wang, Y.; Choi, H.K.; Brinckmann, J.A.; Jiang, X.; Huang, L. Chemical analysis of Panax quinquefolius (North American ginseng): A review. J. Chromatogr. A 2015, 1426, 1–15.
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry 2011, 72, 689–699.
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J. Ginseng Res. 2018, 42, 123–132.
- Liu, L.; Anderson, G.A.; Fernandez, T.G.; Doré, S. Efficacy and mechanism of Panax ginseng in experimental stroke. Front. Neurosci. 2019, 2013, 1–20.
- Nakhjavani, M.; Hardingham, J.E.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Townsend, A.R. Ginsenoside Rg3: Potential molecular targets and therapeutic indication in metastatic breast cancer. Medicines 2019, 6, 17.
- Yu, C.; Wang, C.Z.; Zhou, C.J.; Wang, B.; Han, L.; Zhang, C.F.; Wu, X.H.; Yuan, C.S. Adulteration and cultivation region identification of American ginseng using HPLC coupled with multivariate analysis. J. Pharm. Biomed. Anal. 2014, 99, 8–15.
- Lee, D.G.; Jang, S.I.; Kim, Y.R.; Yang, K.E.; Yoon, S.J.; Lee, Z.W.; An, H.J.; Jang, I.S.; Choi, J.S.; Yoo, H.S. Anti-proliferative effects of ginsenosides extracted from mountain ginseng on lung cancer. Chin. J. Integr. Med. 2016, 2, 344–352.
- Yang, L.; Yu, Q.T.; Ge, Y.Z.; Zhang, W.S.; Fan, Y.; Ma, C.W.; Liu, Q.; Qi, L.W. Distinct urine metabolome after Asian ginseng and American ginseng intervention based on GC-MS metabolomics approach. Sci. Rep. 2018, 6, 39045.
- Lee, O.R.; Nguyen, N.Q.; Lee, K.H.; Kim, Y.C.; Seo, J. Cytohistological study of the leaf structures of Panax ginseng Meyer and Panax quinquefolius L. J. Ginseng Res. 2016, 41, 463–468.
- Pan, Y.; Wang, X.; Sun, G.; Li, F.; Gong, X. Application of RAD sequencing for evaluating the genetic diversity of domesticated Panax notoginseng (Araliaceae). PloS ONE 2016, 11, e0166419.
- Cruse-Sanders, J.M.; Hamrick, J.L. Genetic diversity in harvested and protected populations of wild American ginseng, Panax quinquefolius L. (Araliaceae). Am. J. Bot. 2004, 91, 540–548.
- Jia, L.; Zhao, Y. Current evaluation of the millennium phytomedicine-ginseng (I): Etymology, pharmacognosy, phytochemistry, market and regulations. Curr. Med. Chem. 2009, 16, 2475–2484.
- Qin, Z.; Jia, C.; Liao, D.; Chen, X.; Li, X. Comparison of serum metabolite changes of radiated mice administered with Panax quinquefolium from different cultivation regions using UPLC-Q/TOF-MS based metabolomic approach. Molecules 2018, 23, 1014.
- Souther, S.; Lechowicz, M.J.; McGraw, J.B. Experimental test for adaptive differentiation of ginseng populations reveals complex response to temperature. Ann. Bot. 2012, 110, 829–837.
- Pengelly, A.; Bennett, K. Appalachian Plant Monographs: Panax quinquefolius L., American Ginseng. Available online: http://www.frostburg.edu/aces/appalachian-plants/ (accessed on 5 January 2019).
- Lim, W.; Mudge, K.W.; Vermeylen, F. Effects of population, age and cultivation methods on ginsenoside content of wild American ginseng (Panax quinquefolium). J. Agric. Food Chem. 2005, 53, 8498–8505.
- Proctor, J.T.; Shelp, B.J. Effect of boron nutrition on American ginseng in field and in nutrient cultures. J. Ginseng Res. 2013, 38, 73–77.
- Lee, J.S.; Bae, I. Quality Characteristics, changes in physiochemical properties and functional properties of camembert cheese containing red ginseng powder. Korean J. Food Sci. Anim. Resour. 2018, 38, 64–77.
- Kim, K.T.; Yoo, K.M.; Lee, J.W.; Eom, S.H.; Hwang, I.K.; Lee, C.Y. Protective effect of steamed American ginseng (Panax quinquefolius L.) on V79-4 cells induced by oxidative stress. J. Ethnopharmacol. 2007, 111, 443–450.
- Dolot, M.; Smigielski, K.; Wesolowska, M. Analysis of chosen nutrients in American ginseng (Panax quinquefolium L.) cultivated in Poland. Sci. Pap. Tech. Univ. Lodz Food Chem. Biotechnol. 2006, 70, 53–63.
- Sengupta, S.; Toh, S.A.; Sellers, L.A.; Skepper, J.N.; Koolwijk, P.; Leung, H.W.; Yeung, H.W.; Wong, R.N.; Sasisekharan, R.; Fan, T.P. Modulating angiogenesis: The yin and the yang in ginseng. Circulation 2004, 110, 1219–1225.
- Jung, J.; Kim, K.H.; Yang, K.; Bang, K.H.; Yang, T.J. Practical application of DNA markers for high-throughput authentication of Panax ginseng and Panax quinquefolius from commercial ginseng products. J. Ginseng Res. 2014, 38, 123–129.
- Ma, Z.N.; Li, Y.Z.; Li, W.; Yan, X.T.; Yang, G.; Zhang, J.; Zhao, L.C.; Yang, L.M. Nephroprotective effects of saponins from leaves of Panax quinquefolius against cisplatin-induced acute kidney injury. Int. J. Mol. Sci. 2017, 18, 1407.
- Nag, S.A.; Qin, J.; Wang, W.; Wang, M.H.; Wang, H.; Zhang, R. Ginsenosides as anticancer agents: In Vitro and in vivo activities, structure–activity relationships and molecular mechanisms of action. Front. Pharmacol. 2012, 3, 25.
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Lipert, A.; Szymańska, G. Yeast extract stimulates ginsenoside production in hairy root cultures of American ginseng cultivated in shake flasks and nutrient sprinkle bioreactors. Molecules 2017, 22, 880.
- Feng, R.; Liu, J.; Wang, Z.; Zhang, J.; Cates, C.; Rousselle, T.; Meng, Q.; Li, J. The structure-activity relationship of ginsenosides on hypoxia-reoxygenation induced apoptosis of cardiomyocytes. Biochem. Biophys. Res. Commun. 2017, 494, 556–568.
- Yang, W.Z.; Hu, Y.; Wu, W.Y.; Ye, M.; Guo, D.A. Saponins in the genus Panax L. (Araliaceae): A systematic review of their chemical diversity. Phytochemistry 2014, 106, 7–24.
- Wang, A.B.; Wang, C.Z.; Wu, J.A.; Osinski, J.; Yuan, C.S. Determination of major ginsenosides in Panax quinquefolius (American ginseng) using high-performance liquid chromatography. Phytochem. Anal. 2005, 16, 272–277.
- Shukla, Y.N.; Tripathi, A.K.; Mehta, V.K. Feeding-deterrency of oleanolic acid isolated from Panax quinquefolium against lepidopterans. Phytother. Res. 1997, 11, 591–593.
- Huang, X.; Liu, Y.; Zhang, N.; Sun, X.; Yue, H.; Chen, C.; Liu, S. UPLC Orbitrap HRMS analysis of Panax quinquefolium L. for authentication of Panax genus with chemometric methods. J. Chromatogr. Sci. 2018, 56, 25–35.
- Kim, D.H. Chemical diversity of Panax ginseng, Panax quinquefolium, and Panax notoginseng. J Ginseng Res. 2012, 36, 1–15.
- Liu, Y.Y.; Zhang, T.Y.; Xue, X.; Liu, D.M.; Zhang, H.T.; Yuan, L.L.; Liu, Y.L.; Yang, H.L.; Sun, S.B.; Zhang, C.; et al. Pseudoginsenoside-F11 attenuates cerebral ischemic injury by alleviating autophagic/lysosomal defects. CNS Neurosci. Ther. 2017, 23, 567–579.
- Popovich, D.G.; Yeo, C.R.; Zhang, W. Ginsenosides derived from Asian (Panax ginseng), American ginseng (Panax quinquefolius) and potential cytoactivity. Int. J. Biomed. Pharma. Sci. 2012, 6, 56–62.
- Zhang, X.J.; Huang, L.L.; Cai, X.J.; Li, P.; Wang, Y.T.; Wan, J.B. Fatty acid variability in three medicinal herbs of Panax species. Chem. Cent. J. 2013, 7, 12.
- Wang, M.; Guilbert, L.J.; Li, J.; Wu, Y.; Pang, P.; Basu, T.K.; Shan, J.J. A proprietary extract from north American ginseng (Panax quinquefolium) enhances IL-2 and IFN-gamma productions in murine spleen cells induced by Con-A. Int. Immunopharmacol. 2004, 4, 311–315.
- Wang, L.; Yu, X.; Yang, X.; Li, Y.; Yao, Y.; Lui, E.M.K.; Ren, G. Structural and anti-inflammatory characterization of a novel neutral polysaccharide from North American ginseng (Panax quinquefolius). Int. J. Biol. Macromol. 2015, 74, 12–17.
- Ludwiczuk, A.; Wolski, T.; Hołderna-Kędzia, E. Estimation of the chemical composition and antimicrobial and antioxidant activity of extracts received from leaves and roots of American ginseng (Panax quinquefolium L.). Herba Pol. 2006, 52, 79–90.
- Cui, S.; Wu, J.; Wang, J.; Wang, X. Discrimination of American ginseng and Asian ginseng using electronic nose and gas chromatography mass spectrometry coupled with chemometrics. J. Ginseng Res. 2017, 41, 85–95.
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Szymańska, G.; Wajs-Bonikowska, A.; Bonikowski, R.; Sienkiewicz, M. The increase of triterpene saponin production induced by trans-anethole in hairy root cultures of Panax quinquefolium. Molecules 2018, 23, 2674.
- Wang, C.Z.; Cai, Y.; Anderson, S.; Yuan, C.S. Ginseng metabolites on cancer chemoprevention: An angiogenesis link? Diseases 2015, 3, 193–204.
- Sun, J.; Chen, P. Differentiation of Panax quinquefolius grown in the USA and China using LC/MS-based chromatographic fingerprinting and chemometric approaches. Anal. Bioanal. Chem. 2011, 399, 1877–1889.
- Sun, X.; Chen, P.; Cook, S.L.; Jackson, G.P.; Harnly, J.M.; Harrington, P.B. Classification of cultivation locations of Panax quinquefolius L samples using high performance liquid chromatography-electrospray ionization mass spectrometry and chemometric analysis. Anal. Chem. 2012, 84, 3628–3634.
- Kochan, E.; Kołodziej, B.; Gadomska, G.; Chmiel, A. Content of ginsenosides in Panax quinquefolium from field cultivation. Herba Pol. 2004, 50, 20–27.
- Xiao, D.; Yue, H.; Xiu, Y.; Sun, X.; Wang, Y.B.; Liu, S.Y. Accumulation characteristics and correlation analysis of five ginsenosides with different cultivation ages from different regions. J. Ginseng Res. 2015, 39, 338–344.
- Liu, Y.; Wang, X.; Wang, L.; Chen, X.; Pang, X.; Han, J. A Nucleotide signature for the identification of American ginseng and its products. Front. Plant Sci. 2016, 7, 319.
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 2017, 107, 362–372.
- Wolski, T.; Ludwiczuk, A.; Baj, T.; Głowniak, K.; Świątek, M. Genus Panax—Taxonomy, chemical composition, pharmacological effects, medicinal application and phytochemical analysis of aerial and underground parts of American ginseng—Panax quinquefolium l. Part I. Post. Fitoterapii 2008, 2, 94–114.
- Chen, C.; Chiou, W.; Zhang, J. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol. Sin. 2008, 29, 1103–1108.
- Park, J.D.; Rhee, D.K.; Lee, Y.H. Biological activities and chemistry of saponins from Panax ginseng C.A. Meyer. Phytochem. Rev. 2005, 4, 159–175.
- Rokot, N.T.; Kairupan, T.S.; Cheng, C.-H.; Runtuwene, J.; Kapantow, N.H.; Amitani, M.; Morinaga, A.; Amitani, H.; Asakawa, A.; Inui, A. A Role of ginseng and its constituents in the treatment of central nervous system disorders. Evid. Based Complement. Alternat. Med. 2016, 2016, 2614742.
- Popovich, D.G.; Kitts, D.D. Generation of ginsenosides Rg3 and Rh2 from North American ginseng. Phytochemistry 2004, 65, 337–344.
- Rasheed, N.; Tyagi, E.; Ahmad, A.; Siripurapu, K.B.; Lahiri, S.; Shukla, R.; Palit, G. Involvement of monoamines and proinflammatory cytokines in mediating the anti-stress effects of Panax quinquefolium. J. Ethnopharmacol. 2008, 117, 257–262.
- Xu, H.; Yu, X.; Qu, S.; Chen, Y.; Wang, Z.; Sui, D. In vive and in vitro cardioprotective effects of Panax quinquefolium 20(S)-protopanaxadiol saponins (PQDS), isolated from Panax quinquefolium. Pharmazie 2013, 68, 287–292.
- Wang, M.; Guilbert, L.J.; Ling, L.; Li, J.; Wu, Y.; Xu, S.; Pang, P.; Shan, J.J. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium). J. Pharma Pharmacol. 2001, 53, 1515–1523.
- Xie, G.; Wang, C.Z.; Yu, C.; Qiu, Y.; Wen, X.D.; Zhang, C.F.; Yuan, C.S.; Jia, W. Metabonomic profiling reveals cancer chemopreventive effects of American ginseng on colon carcinogenesis in Apc(Min/+) mice. J. Proteome Res. 2015, 14, 3336–3347.
- Kochan, E.; Wasiela, M.; Sienkiewicz, M. The production of ginsenosides in hairy root cultures of American ginseng, Panax quinquefolium L. and their antimicrobial activity. In Vitro Cell Dev. Biol. Plant 2012, 49, 24–29.
- Sienkiewicz, M.; Głowacka, A.; Kowalczyk, E.; Kochan, E. The activity of different extracts from Panax quinquefolium L. cultures against pathogenic Staphylococcus aureus with respect to ginsenoside content. Arch. Biol. Sci. 2015, 67, 1277–1284.
- Wang, L.; Yang, X.; Yu, X.; Yao, Y.; Ren, G. Evaluation of antibacterial and anti-inflammatory activities of less polar ginsenosides produced from polar ginsenosides by heat-transformation. J. Agric. Food Chem. 2013, 61, 12274–12282.
- Shin, K.; Guo, H.; Cha, Y.; Ban, Y.H.; Seo, D.W.; Choi, Y.; Kim, T.S.; Lee, S.P.; Kim, J.C.; Choi, E.K.; et al. Cereboost™ an American ginseng extract improves cognitive function via up-regulation of choline acetyltransferase expression and neuroprotection. Regul. Toxicol. Pharmacol. 2016, 78, 53–58.
- Alipour, M.; Omri, A.; Suntres, Z.E. Ginseng aqueous extract attenuates the production of virulence factors, stimulates twitching and adhesion, and eradicates biofilms of Pseudomonas aeruginosa. Can. J. Physiol. Pharmacol. 2011, 89, 419–427.
- Xue, P.; Yao, Y.; Yang, X.S.; Feng, J.; Ren, G.X. Improved antimicrobial effect of ginseng extract by heat transformation. J. Ginseng Res. 2017, 41, 180–187.
- Wang, H.X.; Ng, T.B. Quinqueginsin, a novel protein with anti-human immunodeficiency virus, antifungal, ribonuclease and cell-free translation-inhibitory activities from American ginseng roots. Biochem. Biophys. Res. Commun. 2000, 269, 203–208.
- Wang, C.Z.; Du, G.J.; Zhang, Z.; Wen, X.D.; Calway, T.; Zhen, Z.; Musch, M.W.; Bissonnette, M.; Chang, E.B.; Yuan, C.S. Ginsenoside compound K, not Rb1, possesses potential chemopreventive activities in human colorectal cancer. Int. J. Oncol. 2012, 40, 1970–1976.
- Kim, D.H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J. Ginseng Res. 2017, 42, 255–263.
- Wan, J.Y.; Liu, P.; Wang, H.Y.; Qi, L.W.; Wang, C.Z.; Li, P.; Yuan, C.S. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2013, 1286, 83–92.
- Wan, J.Y.; Wang, C.Z.; Liu, Z.; Zhang, Q.H.; Musch, M.W.; Bissonnette, M.; Chang, E.B.; Li, P.; Qi, L.W.; Yuan, C.S. Determination of American ginseng saponins and their metabolites in human plasma, urine and feces samples by liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1015–1016, 62–73.
- Cimo, A.; Soltani, M.; Lui, E.; Hekmat, S. Fortification of probiotic yogurt with ginseng (Panax quinquefolius) extract. J. Food Nutr. Disor. 2013, 2, 2.
- Wang, C.Z.; Yu, C.; Wen, X.D.; Chen, L.; Zhang, C.F.; Calway, T.; Qiu, Y.; Wang, Y.; Zhang, Z.; Anderson, S.; et al. American ginseng attenuates colitis-associated colon carcinogenesis in mice: Impact on gut microbiota and metabolomics. Cancer Prev. Res. 2016, 9, 803–811.
