You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Amina Yu.
Vascular endothelial growth factor (VEGF) is a major therapeutic target for blood–retina barrier (BRB) breakdown in diabetic retinopathy (DR), age-related macular degeneration (AMD), and other hypoxic retinal vascular disorders. VEGF is a direct functional regulator of photoreceptors and VEGF up-regulation in DR is a contributing factor to diabetes-induced alteration of photoreceptor function. This information is critical to the understanding of the therapeutic effect and to the care of anti-VEGF drug-treated patients for BRB breakdown in DR, AMD, and other hypoxic retinal vascular disorders.
- VEGF
- photoreceptors
- neuronal function
- ERG
- diabetic retinopathy
1. Introduction
Diabetic retinopathy (DR), a leading cause of blindness in the developed countries, is regarded as a diabetes-induced disorder of retinal neurons and blood–retina barriers (BRBs). Previous studies have demonstrated that the changes in retinal function and neuronal viability occur before the onset of BRB abnormalities in diabetic patients and animal models [1][2][3][4][5]. In both diabetic humans and animals, retinal function is impaired [6], as measured by changes in electroretinography (ERG) components. The viability of retinal neurons is reduced during early diabetes in streptozotocin-injected rodent models, as demonstrated by increasing neuronal apoptosis and retinal thinning, including the thinning of the inner nuclear layer (INL) and outer nuclear layer (ONL) [1][5]. These results indicate that diabetes induces the loss of neurons in all retinal layers, including photoreceptors. Diabetic patients also demonstrate a loss in green–blue color discrimination, early transition times in dark adaptation, and S-cone sensitivity [3][4]. While these observations are impactful to the field, the mechanisms of diabetes-induced alterations of visual function remain largely uninvestigated, except formulating a clear consensus that diabetes induced alterations of visual function may act as neuronal survival-dependent or -independent fashion [3][4][7].
A major accomplishment in retinal pathobiology in recent decades is the identification of vascular endothelial growth factor (VEGF or VEGF-A) as a cardinal pathogenic factor in retinal neovascularization and vascular leakage in DR and retinopathy of prematurity (ROP) and in choroidal neovascularization in age-related macular degeneration (AMD) [8][9][10][11][12], which leads to the development of anti-VEGF drugs as a major therapeutic strategy for DR, AMD, ROP, and other hypoxic ocular vascular disorders. While VEGF has been shown to act as a positive or negative regulator for neuronal function directly in the brain and peripheral neurons [13][14][15][16][17], its role as a direct functional regulator of retinal neurons has never been addressed. Potentially, elevated VEGF levels could upregulate its downstream signaling cascade and play an important role in altering neuronal function in the retina during the progression of diabetes, such as impairing visual function through alteration of visual cycle and phototransduction machinery in DR [18]. This assumption is supported by well-established observations that both VEGF receptor-1 (VEGFR1) and VEGFR2 are present in the neural retina, including photoreceptors [19][20], in which the role of VEGF as a trophic factor for retinal neurons under stress conditions has been identified [7][20][21][22][23]. To determine if VEGF plays a direct role in regulating photoreceptor function, we developed a procedure to evaluate the immediate effect of rVEGF on retinal neuronal function with ERG, based on similar approaches used in dissecting the direct role of VEGF in neuronal function in the rat hippocampus [14]. This article summarizes our work in revealing the role of VEGF in photoreceptor function using wildtype (WT) or Akita spontaneous diabetic mice [24].
2. Effect of VEGF on Phtoreceptor Function
To determine if VEGF-A or VEGF played a directly role in regulating photoreceptor function, we intravitreally injected human rVEGF to overnight dark-adopted C57BL6 background mice (1.5-mo-old) under long-wavelength illumination and recorded ERG shortly after the injection (20 min, for sufficient diffusion of injected rVEGF). While rVEGF did not appear to alter the time to reach the trough or peak for both scotopic ERG a-wave and b-wave, rVEGF-injected animals demonstrated a dose-dependent (0.1, 0.3, or 0.5 µg rVEGF/eye) reduction of both scotopic ERG a-wave and b-wave amplitudes (Figure 1), suggesting that rVEGF was capable of downregulating rod photoreceptor function immediately after it diffused to photoreceptors and retinal neurons. The relative rVEGF-altered changes in scotopic ERG a-wave and b-wave amplitudes were reduced with time (data not shown). This observation supports that the VEGF-downregulated scotopic ERG a- and b-wave amplitudes recorded shortly after the intravitreal injection are most likely the primary responses by photoreceptors and other retinal neurons. To determine if VEGF played a directly role in regulating cone photoreceptor function, we also recorded photopic ERG in mice injected with rVEGF intravitreally. The rVEGF-injected animals demonstrated a reduction of photopic ERG b-wave amplitudes in a dose-dependent (0.1, 0.3, or 0.5 µg rVEGF/eye) manner (Figure 2), while there was no apparent change in the time-to-peak for photopic b-wave. This result suggests that VEGF may also play a similar role as a direct regulator of cone photoreceptor function.
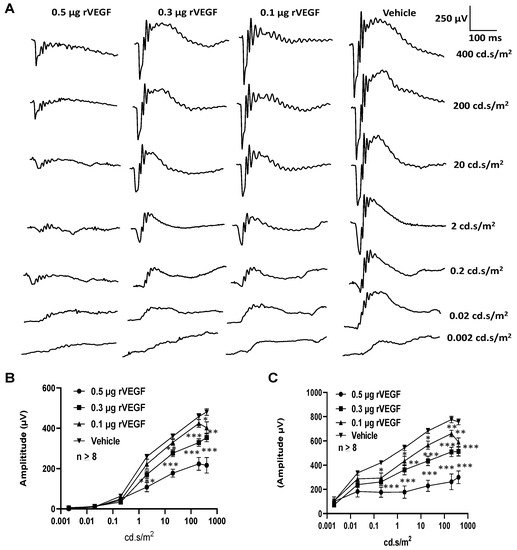
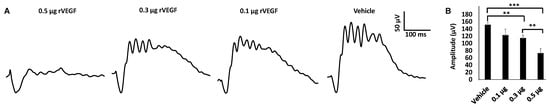

Figure 1. Direct effect of rVEGF on rod photoreceptor function in dark-adapted 1.5-mo-old C57BL6 mice. (A): Representative scotopic ERG tracers with progressive flash intensities from 0.002 to 400 cd·s/m2 in mice 20 min after injected intravitreally with human rVEGF (vehicle, 0.1, 0.3, or 0.5 µg/µL/eye). (B): Analysis of rVEGF-induced reduction of scotopic ERG a-wave amplitudes. (C): Analysis of rVEGF-induced reduction of scotopic ERG b-wave amplitudes. Error bar: SEM (omitted if the value was smaller than the size of the corresponding symbol in the diagram). N > 8 for all data points. ***: p < 0.001; **: p < 0.01; *: p < 0.05. p-Value < 0.05 was considered significant. rVEGF downregulated both scotopic a-wave and b-wave amplitudes in a dose-dependent manner in 1.5 mo-old WT mice.

Figure 2. rVEGF-induced reduction of cone photoreceptor function in 1.5-mo-old C57BL6 mice in a dose-dependent manner. (A) Representative photopic ERG tracers with a flash intensity of 2000 cd·s/m2 after light adaptation at 50 cd·s/m2 for 10 min, which equaled to 30 min after the mice injected with human rVEGF (vehicle, 0.1, 0.3, or 0.5 µg/µL/eye) intravitreally. (B) analysis of rVEGF-induced reduction of photopic ERG b-wave amplitudes. Error bar: SEM. N > 8 for all data points. ***: p < 0.001; **: p < 0.01. p-Value < 0.05 was considered significant. rVEGF downregulated photopic b-wave amplitudes in a dose-dependent manner in 1.5 mo-old WT mice.
3. Effect of VEGF on Phtoreceptor Function in Mouse Model of DR
As VEGF is gradually upregulated in the retina of diabetic mice, we asked the question whether VEGF upregulation is a contributing factor for the reduction of photoreceptor function in diabetes. We examined both scotopic and photopic ERG after intravitreal injection of rVEGF (0.3 µg/eye) or vehicle to 5-mo-old Akita spontaneous diabetic mice, which have been suggested as a suitable model for experimental DR research [24]. As expected, the Akita spontaneous diabetic mice (in C57BL6 background) demonstrated a significant loss of both scotopic ERG a- and b-wave amplitudes compared with age-matched C57BL6 WT counterparts (Figure 3). However, the effect of intravitreally delivered rVEGF on scotopic ERG a- and b-wave amplitudes was diminished in 5-mo-old Akita mice (Figure 3), which was contrary to that in WT control animals that demonstrated a similar reduction of scotopic ERG a- and b-wave amplitudes after intravitreal rVEGF injection (Figure 3), as observed in younger animals (Figure 1). Likewise, the effect of intravitreally delivered rVEGF (0.3 µg/eye) on photopic ERG b-wave amplitude was also diminished in these Akita mice (Figure 4). We then verified if retinal VEGF is gradually upregulated during the progression of diabetes in Akita mice with IHC. While the 5-mo-old WT mice demonstrated VEGF signals in the retinal pigment epithelium (RPE), photoreceptor inner segments (PIS), Müller glia (MG) cell bodies in ONL, outer plexiform (OPL), INL, ganglion cell layer (GCL), the age-matched Akita mice had a significant elevation of retinal VEGF, particularly in areas of the RPE, PIS, MG in ONL, OPL, INL, and GCL. In addition, the number of VEGF-positive cells in INL and GCL were substantially increased (Figure 5, of note, diabetic animals may be more vulnerable for detaching the RPE and sclera from photoreceptor cells due to the loss of RPE structural integrity [25]). This result suggests that the elevated level of retinal VEGF is likely a major contributing factor for diabetes-downregulated photoreceptor function in aging Akita mice. To address if VEGF receptors or/and co-receptors regulate neuronal function in photoreceptors, we also perform IHC and found that in addition to the commonly known presence in the RPE, MG, and GCL, VEGFR2, the most likely VEGF receptor for regulating neuronal function, was present in the nuclear envelops of photoreceptors (arrows in ONL), OPL (location of photoreceptor synaptic terminals), and INL neurons (arrows in INL) in 3-mo-old adult mice (Figure 6).
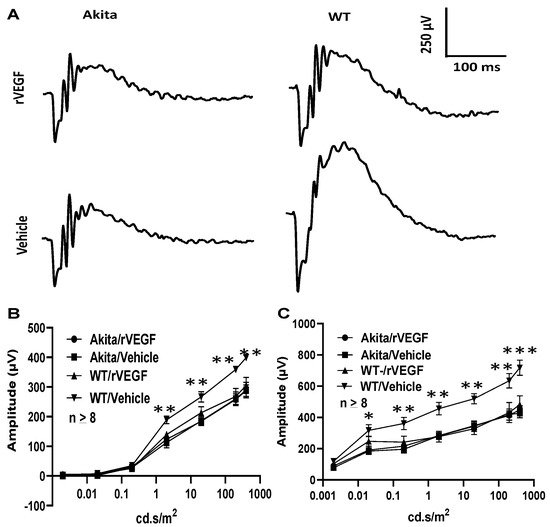
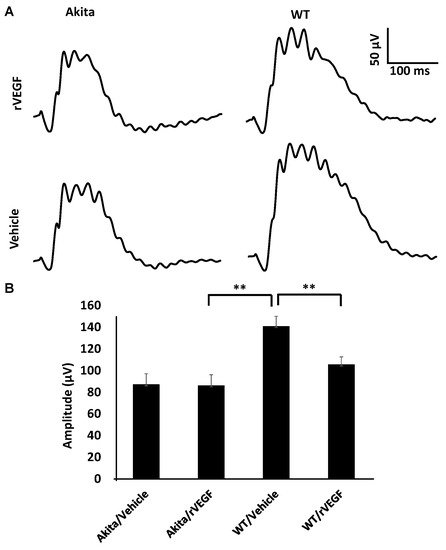
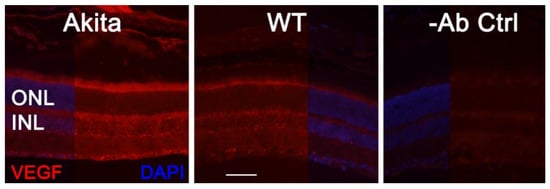
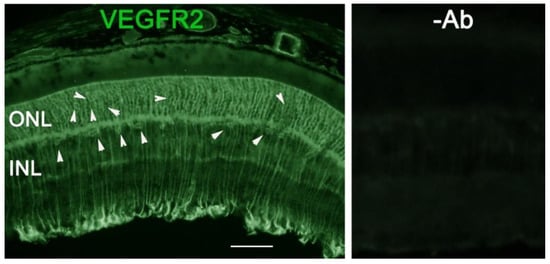

Figure 3. Diabetes nullified the effect of rVEGF-induced reduction of rod photoreceptor function in 5-mo-old male Akita spontaneous diabetes mice (in C57BL6 background). (A) Representative scotopic ERG tracers with flash intensity of 20 cd·s/m2 from age-matched Aktia and C57BL6 control mice 20 min after injected intravitreally with human rVEGF (vehicle or 0.3 µg/µL/eye). (B,C) Analysis of rVEGF-induced reduction of scotopic ERG a- and b-wave amplitudes. Error bar: SEM (omitted if the value was smaller than the size of the corresponding symbol in the diagram). N > 8 for all data points. ***: p < 0.001; **: p < 0.01; *: p < 0.05. p-Value < 0.05 was considered significant. Compared with WT controls, the effect of rVEGF downregulated both scotopic a-wave and b-wave amplitudes was diminished in 5-mo-old male Akita spontaneous diabetes mice.

Figure 4. Diabetes nullified the effect of rVEGF-induced alteration of cone photoreceptor function in 5-mo-old male Akita spontaneous diabetes mice in C57BL6 background. (A) Representative photopic ERG tracers with a flash intensity of 2000 cd·s/m2 after light adaptation at 50 cd·s/m2 for 10 min, which equaled to 30 min after the mice injected with human rVEGF (vehicle or 0.3 µg/µL/eye) intravitreally. (B) Analysis of rVEGF-induced reduction of photopic ERG b-wave amplitudes. Error bar: SEM. N > 8 for all data points. **: p < 0.01. p-Value < 0.05 was considered significant. Compared with WT controls, the effect of rVEGF downregulated photopic ERG b-wave amplitudes was diminished in Akita spontaneous diabetes mice.

Figure 5. IHC detection of retinal VEGF distribution in 5-mo-old Akita spontaneous diabetes mice in (C57BL6 background) and WT controls. Scale bar: 50 µm. VEGF levels were significantly increased in the retina, particularly in the RPE, PISs, MG cell bodies, INL, ONL, and GCL. The number of VEGF-positive cells in the INL and GCL was substantially increased.

Figure 6. IHC detection of retinal VEGFR2 distribution in 3-mo-old C57BL6 background mice. Scale bar: 50 µm. In addition to the commonly known presence in the RPE, MG, and GCL, VEGFR2 was present in photoreceptor nuclear envelops (arrows in ONL), OPL (location of photoreceptor synaptic terminals), and INL neurons (arrows in INL) in adult WT mice.
References
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of retinal neurons in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3330–3336.
- Gardner, T.W.; Antonetti, D.A.; Barber, A.J.; LaNoue, K.F.; Nakamura, M. New insights into the pathophysiology of diabetic retinopathy: Potential cell-specific therapeutic targets. Diabetes Technol. Ther. 2000, 2, 601–608.
- Terasaki, H.; Hirose, H.; Miyake, Y. S-cone pathway sensitivity in diabetes measured with threshold versus intensity curves on flashed backgrounds. Investig. Ophthalmol. Vis. Sci. 1996, 37, 680–684.
- Aspinall, P.A. Rod-cone interaction: Some indirect evidence. Acta Ophthalmol. 1977, 55, 294–302.
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791.
- Juen, S.; Kieselbach, G.F. Electrophysiological changes in juvenile diabetics without retinopathy. Arch. Ophthalmol. 1990, 108, 372–375.
- Fu, S.; Dong, S.; Zhu, M.; Sherry, D.M.; Wang, C.; You, Z.; Haigh, J.J.; Le, Y.Z. Müller glia are a major cellular source of survival signals for retinal neurons in diabetes. Diabetes 2015, 64, 3554–3563.
- Bai, Y.; Ma, J.X.; Guo, J.; Wang, J.; Zhu, M.; Chen, Y.; Le, Y.Z. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J. Pathol. 2009, 219, 446–454.
- Wang, J.; Xu, X.; Elliott, M.H.; Zhu, M.; Le, Y.Z. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 2010, 59, 2297–2305.
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487.
- Alon, T.; Hemo, I.; Itin, A.; Pe’er, J.; Stone, J.; Keshet, E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1995, 1, 1024–1028.
- Wang, J.J.; Zhu, M.; Le, Y.Z. Functions of Muller cell-derived vascular endothelial growth factor in diabetic retinopathy. World J. Diabetes 2015, 6, 726–733.
- McCloskey, D.P.; Hintz, T.M.; Scharfman, H.E. Modulation of vascular endothelial growth factor (VEGF) expression in motor neurons and its electrophysiological effects. Brain Res. Bull. 2008, 76, 36–44.
- McCloskey, D.P.; Croll, S.D.; Scharfman, H.E. Depression of synaptic transmission by vascular endothelial growth factor in adult rat hippocampus and evidence for increased efficacy after chronic seizures. J. Neurosci. 2005, 25, 8889–8897.
- Kim, B.W.; Choi, M.; Kim, Y.S.; Park, H.; Lee, H.R.; Yun, C.O.; Kim, E.J.; Choi, J.S.; Kim, S.; Rhim, H.; et al. Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal. 2008, 20, 714–725.
- Lin, J.; Li, G.; Den, X.; Xu, C.; Liu, S.; Gao, Y.; Liu, H.; Zhang, J.; Li, X.; Liang, S. VEGF and its receptor-2 involved in neuropathic pain transmission mediated by P2X(2)(/)(3) receptor of primary sensory neurons. Brain Res. Bull. 2010, 83, 284–291.
- Bouzioukh, F.; Daoudal, G.; Falk, J.; Debanne, D.; Rougon, G.; Castellani, V. Semaphorin3A regulates synaptic function of differentiated hippocampal neurons. Eur. J. Neurosci. 2006, 23, 2247–2254.
- Malechka, V.V.; Moiseyev, G.; Takahashi, Y.; Shin, Y.; Ma, J.X. Impaired Rhodopsin Generation in the Rat Model of Diabetic Retinopathy. Am. J. Pathol. 2017, 187, 2222–2231.
- Shih, S.C.; Ju, M.; Liu, N.; Smith, L.E. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. J. Clin. Investig. 2003, 112, 50–57.
- Saint-Geniez, M.; Maharaj, A.S.; Walshe, T.E.; Tucker, B.A.; Sekiyama, E.; Kurihara, T.; Darland, D.C.; Young, M.J.; D’Amore, P.A. Endogenous VEGF is required for visual function: Evidence for a survival role on muller cells and photoreceptors. PLoS ONE 2008, 3, e3554.
- Nishijima, K.; Ng, Y.S.; Zhong, L.; Bradley, J.; Schubert, W.; Jo, N.; Akita, J.; Samuelsson, S.J.; Robinson, G.S.; Adamis, A.P.; et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 2007, 171, 53–67.
- Le, Y.Z. VEGF production and signaling in Müller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases. Vis. Res. 2017, 139, 108–114.
- Le, Y.Z.; Xu, B.; Chucair-Elliott, A.J.; Zhang, H.; Zhu, M. VEGF mediates retinal Müller cell viability and neuroprotection through BDNF in diabetes. Biomolecules 2021, 11, 712.
- Barber, A.J.; Antonetti, D.A.; Kern, T.S.; Reiter, C.E.; Soans, R.S.; Krady, J.K.; Levison, S.W.; Gardner, T.W.; Bronson, S.K. The Ins2Akita mouse as a model of early retinal complications in diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2210–2218.
- Xu, H.Z.; Le, Y.Z. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2160–2164.
More
