Many organic pollutants are discharged into the environment, which results in the frequent detection of organic pollutants in surface water and underground water. Some of the organic pollutants can stay for a long time in the environment due to their recalcitrance. Advanced oxidation processes (AOPs) can effectively treat the recalcitrant organic compounds in water. Photocatalysis as one of the AOPs has attracted a lot of interest. BiOCl and g-C3N4 are nice photocatalysts. However, their catalytic activity should be further improved for industrial utilization. The construction of heterojunction between the two different components is deemed as an efficient strategy for developing a highly efficient photocatalyst. As a typical type-II heterojunction, g-C3N4/BiOCl heterojunctions showed better photocatalytic performance. To date, the g-C3N4/BiOCl composites were mainly studied in the field of water purification. The photoactivity of the pristine catalysts was greatly enhanced by the combination of the two materials. However, three kinds of proposed mechanisms were used to explain the improvement of the g-C3N4/BiOCl heterojunctions. But few researchers tried to explain why there were three different scenarios employed to explain the charge transfer. According to the articles reviewed, no direct evidence could indicate whether the band structures of the heterojunctions based on BiOCl and g-C3N4 were changed. Therefore, many more studies are needed to reveal the truth. Having a clearer understanding of the mechanism is beneficial for researchers to construct more efficient photocatalysts. This article is trying to start a new direction of research to inspire more researchers to prepare highly effective photocatalysts.
- BiOCl
- C3N4
- photocatalysis
- mechanism
1. Introduction
2. Mechanisms of the BiOCl/g-C3N4 Heterojunctions
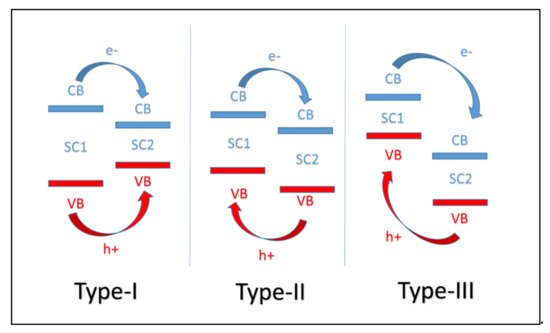
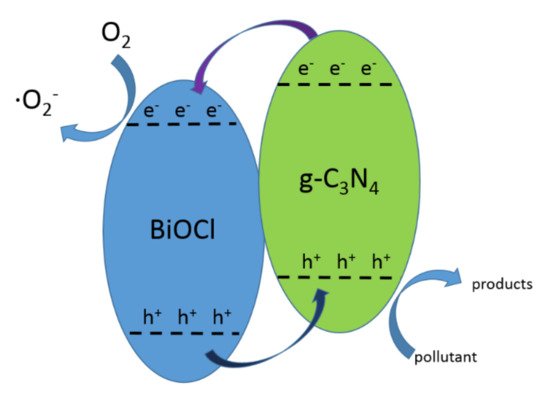
42.1. CNB Heterojunction
References
- Pan, M.; Wong, C.K.C.; Chu, L.M. Distribution of Antibiotics in Wastewater-Irrigated Soils and Their Accumulation in Vegetable Crops in the Pearl River Delta, Southern China. J. Agric. Food Chem. 2014, 62, 11062–11069.
- Sharma, V.; Anquandah, G.A.K.; Yngard, R.A.; Kim, H.; Fekete, J.; Bouzek, K.; Ray, A.K.; Golovko, D. Nonylphenol, octylphenol, and bisphenol-A in the aquatic environment: A review on occurrence, fate, and treatment. J. Environ. Sci. Health Part A 2009, 44, 423–442.
- Yesilada, O.; Asma, D.; Cing, S. Decolorization of textile dyes by fungal pellets. Process. Biochem. 2003, 38, 933–938.
- Ahern, J.; Fairchild, R.; Thomas, J.S.; Carr, J.; Patterson, H.H. Characterization of BiOX compounds as photocatalysts for the degradation of pharmaceuticals in water. Appl. Catal. B Environ. 2015, 179, 229–238.
- Sun, S.; Wang, W.; Zhang, L.; Zhou, L.; Yin, W.; Shang, M. Visible Light-Induced Efficient Contaminant Removal by Bi5O7I. Environ. Sci. Technol. 2009, 43, 2005–2010.
- Chang, F.; Xie, Y.; Zhang, J.; Chen, J.; Li, C.; Wang, J.; Luo, J.; Deng, B.; Hu, X. Construction of exfoliated g-C3N4 nanosheets–BiOCl hybrids with enhanced photocatalytic performance. RSC Adv. 2014, 4, 28519–28528.
- Zhou, C.; Lai, C.; Xu, P.; Zeng, G.; Huang, D.; Li, Z.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; et al. Rational Design of Carbon-Doped Carbon Nitride/Bi12O17Cl2 Composites: A Promising Candidate Photocatalyst for Boosting Visible-Light-Driven Photocatalytic Degradation of Tetracycline. ACS Sustain. Chem. Eng. 2018, 6, 6941–6949.
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management. Adv. Colloid Interface Sci. 2018, 254, 76–93.
- Liu, Y.; Zhang, H.; Ke, J.; Zhang, J.; Tian, W.; Xu, X.; Duan, X.; Sun, P.H.; Tade, M.; Wang, S. 0D (MoS2)/2D (g-C3N4) heterojunctions in Z-scheme for enhanced photocatalytic and electrochemical hydrogen evolution. Appl. Catal. B Environ. 2018, 228, 64–74.
- Guo, F.; Shi, W.; Li, M.; Shi, Y.; Wen, H. 2D/2D Z-scheme heterojunction of CuInS2/g-C3N4 for enhanced visible-light-driven photocatalytic activity towards the degradation of tetracycline. Sep. Purif. Technol. 2019, 210, 608–615.
- Liang, S.; Zhang, D.; Pu, X.; Yao, X.; Han, R.; Yin, J.; Ren, X. A novel Ag2O/g-C3N4 p-n heterojunction photocatalysts with enhanced visible and near-infrared light activity. Sep. Purif. Technol. 2019, 210, 786–797.
- Liu, G.; Wang, G.; Hu, Z.; Su, Y.; Zhao, L. Ag2O nanoparticles decorated TiO2 nanofibers as a p-n heterojunction for enhanced photocatalytic decomposition of RhB under visible light irradiation. Appl. Surf. Sci. 2019, 465, 902–910.
- Guo, F.; Shi, W.; Wang, H.; Han, M.; Guan, W.; Huang, H.; Liu, Y.; Kang, Z. Study on highly enhanced photocatalytic tetracycline degradation of type II AgI/CuBi2O4 and Z-scheme AgBr/CuBi2O4 heterojunction photocatalysts. J. Hazard. Mater. 2018, 349, 111–118.
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (0 4 0) binary heterojunction photocatalysts with enhanced photocatalytic activity for Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902.
- Zhu, C.; Zhang, L.; Jiang, B.; Zheng, J.; Hu, P.; Li, S.; Wu, M.; Wu, W. Fabrication of Z-scheme Ag3PO4/MoS2 composites with enhanced photocatalytic activity and stability for organic pollutant degradation. Appl. Surf. Sci. 2016, 377, 99–108.
- Hao, Q.; Niu, X.; Nie, C.; Hao, S.; Zou, W.; Ge, J.; Chen, D.; Yao, W. A highly efficient g-C3N4/SiO2 heterojunction: The role of SiO2 in the enhancement of visible light photocatalytic activity. Phys. Chem. Chem. Phys. 2016, 18, 31410–31418.
- Nguyen, T.B.; Huang, C.; Doong, R.-A. Photocatalytic degradation of bisphenol A over a ZnFe2O4/TiO2 nanocomposite under visible light. Sci. Total Environ. 2019, 646, 745–756.
- Luo, J.; Li, R.; Chen, Y.; Zhou, X.; Ning, X.; Zhan, L.; Ma, L.; Xu, X.; Xu, L.; Zhang, L. Rational design of Z-scheme LaFeO3/SnS2 hybrid with boosted visible light photocatalytic activity towards tetracycline degradation. Sep. Purif. Technol. 2019, 210, 417–430.
- He, R.; Zhou, J.; Fu, H.; Zhang, S.; Jiang, C. Room-temperature in situ fabrication of Bi2O3/g-C3N4 direct Z-scheme photocatalyst with enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 430, 273–282.
- Chen, Y.; Zhu, G.; Hojamberdiev, M.; Gao, J.; Zhu, R.; Wang, C.; Wei, X.; Liu, P. Three-dimensional Ag2O/Bi5O7I p–n heterojunction photocatalyst harnessing UV–vis–NIR broad spectrum for photodegradation of organic pollutants. J. Hazard. Mater. 2018, 344, 42–54.
- Shi, S.; Gondal, M.; Al-Saadi, A.; Fajgar, R.; Kupcik, J.; Chang, X.; Shen, K.; Xu, Q.; Seddigi, Z. Facile preparation of g-C3N4 modified BiOCl hybrid photocatalyst and vital role of frontier orbital energy levels of model compounds in photoactivity enhancement. J. Colloid Interface Sci. 2014, 416, 212–219.
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329.
- Li, Y.; Zhang, C.; Shuai, D.; Naraginti, S.; Wang, D.; Zhang, W. Visible-light-driven photocatalytic inactivation of MS2 by metal-free g-C3N4: Virucidal performance and mechanism. Water Res. 2016, 106, 249–258.
- Goettmann, F.; Fischer, A.; Antonietti, M.; Thomas, A. Metal-free catalysis of sustainable Friedel–Crafts reactions: Direct activation of benzene by carbon nitrides to avoid the use of metal chlorides and halogenated compounds. Chem. Commun. 2006, 4530–4532.
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80.
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974.
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride materials: Controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731.
- Pawar, R.; Kang, S.; Ahn, S.H.; Lee, C.S. Gold nanoparticle modified graphitic carbon nitride/multi-walled carbon nanotube (g-C3N4/CNTs/Au) hybrid photocatalysts for effective water splitting and degradation. RSC Adv. 2015, 5, 24281–24292.
- Lan, M.; Fan, G.; Yang, L.; Li, F. Enhanced visible-light-induced photocatalytic performance of a novel ternary semiconductor coupling system based on hybrid Zn–In mixed metal oxide/g-C3N4 composites. RSC Adv. 2014, 5, 5725–5734.
- Huang, L.; Xu, H.; Li, Y.; Li, H.; Cheng, X.; Xia, J.; Xu, Y.; Cai, G. Visible-light-induced WO3/g-C3N4 composites with enhanced photocatalytic activity. Dalton Trans. 2013, 42, 8606–8616.
- Wang, Y.; Wang, Z.; Muhammad, S.; He, J. Graphite-like C3N4 hybridized ZnWO4 nanorods: Synthesis and its enhanced photocatalysis in visible light. CrystEngComm 2012, 14, 5065–5070.
- Xing, C.; Wu, Z.; Jiang, D.; Chen, M. Hydrothermal synthesis of In2S3/g-C3N4 heterojunctions with enhanced photocatalytic activity. J. Colloid Interface Sci. 2014, 433, 9–15.
- Han, G.; Li, D.Y.; Zheng, Y.F.; Song, X.C. Enhanced Visible-Light-Responsive Photocatalytic Properties of Bi2MoO6-BiOCl Nanoplate Composites. J. Nanosci. Nanotechnol. 2018, 18, 5575–5581.
- Lei, L.; Gao, D.; Jin, H.; Zhang, Q.; Xu, J.; Fu, Z. A novel enhanced visible-light-driven photocatalyst via hybridization of nanosized BiOCl and graphitic C3N4. Dalton Trans. 2015, 44, 795–803.
- Ramírez-Meneses, E.; Valencia-Barrón, J.P.; Hernández-Pérez, M.A.; Domínguez-Crespo, M.A.; Torres, A.; Palacios, E. Synthesis and Characterization of BiOCl Powders with Soft Templates. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2350–2364.
- Lin, W.; Yu, X.; Shen, Y.; Chen, H.; Zhu, Y.; Zhang, Y.; Meng, H. Carbon dots/BiOCl films with enhanced visible light photocatalytic performance. J. Nanoparticle Res. 2017, 19, 56.
- Singh, S.; Sharma, R.; Khanuja, M. A review and recent developments on strategies to improve the photocatalytic elimination of organic dye pollutants by BiOX (X=Cl, Br, I, F) nanostructures. Korean J. Chem. Eng. 2018, 35, 1955–1968.
- Yu, C.L.; Chen, J.C.; Zhou, W.Q.; Wei, L.F.; Fan, Q.Z. Grinding calcination preparation of WO3/BiOCl heterostructures with enhanced visible light photocatalytic activity. Mater. Res. Innov. 2014, 19, 54–59.
- Cui, Z.; Song, H.; Ge, S.; He, W.; Liu, Y. Fabrication of BiOCl/BiOBr hybrid nanosheets with enhanced superoxide radical dominating visible light driven photocatalytic activity. Appl. Surf. Sci. 2019, 467, 505–513.
- Junxiu, W.; Zhenzong, Z.; Xi, W.; Yi, S.; Yongfu, G.; Keung, W.P.; Renbi, B. Synthesis of novel p-n heterojunction m-Bi2O4/BiOCl nanocomposite with excellent photocatalytic activity through ion-etching method. Chin. J. Catal. 2018, 39, 1792–1803.
- Song, L.; Pang, Y.; Zheng, Y.; Chen, C.; Ge, L. Design, preparation and enhanced photocatalytic activity of porous BiOCl/BiVO4 microspheres via a coprecipitation-hydrothermal method. J. Alloys Compd. 2017, 710, 375–382.
- Yu, L.; Zhang, X.; Li, G.; Cao, Y.; Shao, Y.; Li, D. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B Environ. 2016, 187, 301–309.
- Zhong, Y.; Liu, Y.; Wu, S.; Zhu, Y.; Chen, H.; Yu, X.; Zhang, Y. Facile Fabrication of BiOI/BiOCl Immobilized Films with Improved Visible Light Photocatalytic Performance. Front. Chem. 2018, 6, 58.
- Wang, X.J.; Wang, Q.; Li, F.-T.; Yang, W.-Y.; Zhao, Y.; Hao, Y.-J.; Liu, S.-J. Novel BiOCl–C3N4 heterojunction photocatalysts: In situ preparation via an ionic-liquid-assisted solvent-thermal route and their visible-light photocatalytic activities. Chem. Eng. J. 2013, 234, 361–371.
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479.
- Liu, S.; Liu, Y.; Dai, G.; Bao, X.; Huang, N.; Peng, R.; Zhou, Y. Synthesis and characterization of novel Bi2S3/BiOCl/g-C3N4 composite with efficient visible-light photocatalytic activity. Mater. Lett. 2019, 241, 190–193.
- Dong, X.; Sun, Z.; Zhang, X.; Li, C.; Zheng, S. Construction of BiOCl/g-C3N4/kaolinite composite and its enhanced photocatalysis performance under visible-light irradiation. J. Taiwan Inst. Chem. Eng. 2018, 84, 203–211.
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A.; Yubuta, K. Novel g-C3N4 nanosheets/CDs/BiOCl photocatalysts with exceptional activity under visible light. J. Am. Ceram. Soc. 2018, 102, 1435–1453.
- Zhao, S.; Zhang, Y.; Zhou, Y.; Fang, J.; Wang, Y.; Zhang, C.; Chen, W. Fabrication of sandwich-structured g-C3N4/Au/BiOCl Z-scheme photocatalyst with enhanced photocatalytic performance under visible light irradiation. J. Mater. Sci. 2018, 53, 6008–6020.
- Xue, J.; Li, X.; Ma, S.; Xu, P.; Wang, M.; Ye, Z. Facile fabrication of BiOCl/RGO/protonated g-C3N4 ternary nanocomposite as Z-scheme photocatalyst for tetracycline degradation and benzyl alcohol oxidation. J. Mater. Sci. 2018, 54, 1275–1290.
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440.
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244.
- AlMarzouqi, F.; Al Farsi, B.; Kuvarega, A.T.; Al Lawati, H.A.J.; Al Kindy, S.M.Z.; Kim, Y.; Selvaraj, R. Controlled Microwave-Assisted Synthesis of the 2D-BiOCl/2D-g-C3N4 Heterostructure for the Degradation of Amine-Based Pharmaceuticals under Solar Light Illumination. ACS Omega 2019, 4, 4671–4678.
- Yang, Y.; Zhou, F.; Zhan, S.; Liu, Y.; Yin, Y. Enhanced Photocatalytic Activity of BiOCl Hybridized with g-C3N4. J. Inorg. Organomet. Polym. Mater. 2016, 26, 91–99.
- Liu, W.; Qiao, L.; Zhu, A.; Liu, Y.; Pan, J. Constructing 2D BiOCl/C3N4 layered composite with large contact surface for visible-light-driven photocatalytic degradation. Appl. Surf. Sci. 2017, 426, 897–905.
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: A critical review. Catal. Today 2019, 335, 65–77.
- Song, L.; Zheng, Y.; Chen, C. Sonication-assisted deposition–precipitation synthesis of graphitic C3N4/BiOCl heterostructured photocatalysts with enhanced rhodamine B photodegradation activity. J. Mater. Sci. Mater. Electron. 2017, 28, 15861–15869.
- Sun, J.; Song, J.; Gondal, M.A.; Shi, S.; Lu, Z.; Xu, Q.; Chang, X.; Xiang, D.; Shen, K. Preparation of g-C3N4/BiOX (X = Cl, Br, I) composites, and their photocatalytic activity under visible light irradiation. Res. Chem. Intermed. 2014, 41, 6941–6955.
- Li, Q.; Zhao, X.; Yang, J.; Jia, C.-J.; Jin, Z.; Fan, W. Exploring the effects of nanocrystal facet orientations in g-C3N4/BiOCl heterostructures on photocatalytic performance. Nanoscale 2015, 7, 18971–18983.
- Song, L.; Pang, Y.; Zheng, Y.; Ge, L. Hydrothermal synthesis of novel g-C3N4/BiOCl heterostructure nanodiscs for efficient visible light photodegradation of Rhodamine B. Appl. Phys. A 2017, 123, 500.
- Jia, T.; Li, J.; Long, F.; Fu, F.; Zhao, J.; Deng, Z.; Wang, X.; Zhang, Y. Ultrathin g-C3N4 Nanosheet-Modified BiOCl Hierarchical Flower-Like Plate Heterostructure with Enhanced Photostability and Photocatalytic Performance. Crystals 2017, 7, 266.
- Bellamkonda, S.; Rao, G.R. Nanojunction-mediated visible light photocatalytic enhancement in heterostructured ternary BiOCl/ CdS/g-C3N4 nanocomposites. Catal. Today 2019, 321–322, 18–25.
- Aghdam, S.M.; Haghighi, M.; Allahyari, S.; Yosefi, L. Precipitation dispersion of various ratios of BiOI/BiOCl nanocomposite over g-C3N4 for promoted visible light nanophotocatalyst used in removal of acid orange 7 from water. J. Photochem. Photobiol. A Chem. 2017, 338, 201–212.
- Bai, X.; Wang, L.; Wang, Y.; Yao, W.; Zhu, Y. Enhanced oxidation ability of g-C3N4 photocatalyst via C60 modification. Appl. Catal. B Environ. 2014, 152–153, 262–270.
