As climatic conditions are altering on global scale and precipitation is therefore altering due to such weather conditions. Few areas experience high rain, while others perceive lower or very minimal rainfall depending on where there is disaster in the form of either drought or flooding. Overall agriculture faces a huge impact and treatments to such conditions are required. Strategies such as NO-based molecules, H
2S compounds, etc., act as impactful adjuncts. Drought stress has seriously impacted horticultural crops and impediment towards achieving productivity targets
[38][120]. Additionally, limited rainfall and higher evaporation due to enhanced temperature also induces the impact of drought. Therefore, plants possess adaptive measures to survive during such unfavorable situations through regulating stomatal activities by reducing the transpiration rate so as to retain the water within for regulating physiological activities. H
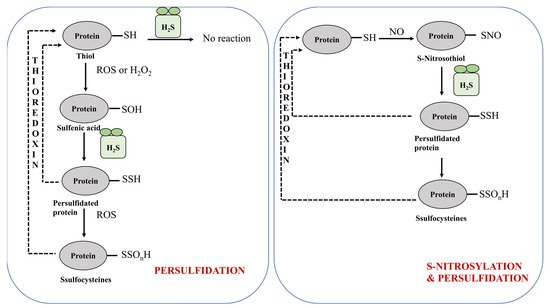
2S also acts up/down stream in NO-signaling pathways, based on activities such as stomatal movement, closure, etc., during stressed conditions
[39][15]. The role of H
2S in stomatal activities has been observed and studies are further conducted to understand its exact mechanism. To elucidate, H
2S causes stomatal opening and closing under varied conditions in response to adverse conditions. Another study reported that short H
2S-exposure in plants led to induce stomatal closure whereas long exposure led to stimulate stomatal activities and H
2S was also mediated by 8-mercapto-cGMP, respectively
[40][121]. cGMP also acts as a downstream mediator of NO in plants and therefore both of them work in corroboration with one another. H
2S treatment in plants regulates the relative water content of plants subjected to drought, however, the H
2S acts as a donor during such conditions followed by inducing the metabolic profiles of plants in the form of polyamines, glycine betaine, osmolytes, proline and H
2S-biosynthesis
[41][122]. Additionally, genes encoding soluble sugars, aquaporins, polyamines, choline monooxygenases, and betaine aldehyde dehydrogenases, etc., are also upregulated after H
2S application in drought stressed plants
[41][122]. In addition to this, plants with H
2S treatment also reduced oxidative stress markers such as MDA and H
2O
2 [41][122]. NaHS treatment in Bermuda grass also stimulated tolerance against salt, osmotic, and chilling stress and this is mainly due to increased activities of antioxidants and osmolytes
[32][114]. Further, proteomic approaches were used in H
2S-mediated drought resistance. They reported the imperative role of proteins namely, S-nitrosated proteins, photosynthetic proteins, etc., induced by H
2S. Henceforth, the plant-water relations, plant movements, stomatal opening/closing, etc., act as suitable target sites for H
2S for modulating different physiological activities in plants. H
2S-formulations act as the most suitable molecules for stress resistance in plants.