Antimicrobial resistance (AMR) is one of the top 10 global public health threats facing humanity, especially in low-resource settings, and requires an interdisciplinary response across academia, government, countries, and societies. If unchecked, AMR will hamper progress towards reaching the United Nations Sustainable Development Goals (SDGs), including ending poverty and hunger, promoting healthy lives and well-being, and achieving sustained economic growth. There are many global initiatives to curb the effects of AMR, but significant gaps remain. New ways of thinking and operating in the context of the SDGs are essential to making progress. In this entry, we define the next generation of the AMR research network, its composition, and strategic activities that can help mitigate the threats due to AMR at the local, regional, and global levels. This is supported by a review of recent literature and bibliometric and network analyses to examine the current and future state of AMR research networks for global health and sustainable development.
Antimicrobial resistance (AMR) happens when microorganisms no longer respond to antimicrobials to treat them. Modern medicine depends on these medical substances to protect people against infection. However, several decades of antimicrobial abuse and misuse in humans, animals, and agricultural practices have accelerated AMR and created health emergencies
1. Introduction
Antimicrobial resistance (AMR) happens when microorganisms no longer respond to antimicrobials to treat them. Modern medicine depends on these medical substances to protect people against infection. However, several decades of antimicrobial abuse and misuse in humans, animals, and agricultural practices have accelerated AMR and created health emergencies
with huge socioeconomic impact
. Current interventions exist, yet many are not sufficient to counteract AMR and its drivers, and the AMR community identified this as an ‘implementation gap’
. With new AMR mechanisms spreading globally, generating greater burden in low-resource non-U.S. settings, all these consequences and threats are expected to rise more affecting the delivery of Sustainable Development Goals (SDGs)—the “blueprint to achieve a better and more sustainable future for all”
. Addressing AMR as a global public health and development challenge requires holistic, multi-sectoral, and multi-disciplinary approaches across academic institutions, government sectors, and society
[1]. Sustained collaborations are needed to curb current and future AMR challenges and not to place SDGs at risk. In this entry, we review AMR, define its drivers, and present its impact on SDGs. We also describe current global collaborative efforts on mitigating AMR and corroborate this review with bibliometric and network analyses. We identify gaps and present our definition and views on the next generation of AMR research networks, and what this network model shall look like. We define hallmarks, strategic areas, and phase-gate milestones that countries, institutions, and networks of AMR experts need to invest in to mitigate the effects of AMR as a globally multifaceted phenomenon. . Sustained collaborations are needed to curb current and future AMR challenges and not to place SDGs at risk. In this entry, we review AMR, define its drivers, and present its impact on SDGs. We also describe current global collaborative efforts on mitigating AMR and corroborate this review with bibliometric and network analyses. We identify gaps and present our definition and views on the next generation of AMR research networks, and what this network model shall look like. We define hallmarks, strategic areas, and phase-gate milestones that countries, institutions, and networks of AMR experts need to invest in to mitigate the effects of AMR as a globally multifaceted phenomenon.
2. Drivers of Antimicrobial Resistance
A 2017 report by the National Academies of Sciences, Engineering, and Medicine (NASEM) cites three major drivers that contribute to the emergence and rapid evolution of AMR [5]: (1) inappropriate antimicrobial prescription in healthcare practices; (2) inappropriate use of antimicrobials in livestock; and (3) promulgation of antimicrobial resistance genes in the environment. Many other factors also contribute to the increase in AMR threats and these factors transcend discipline and sectors.
Many findings suggest that inappropriate antimicrobial prescription in healthcare practices (driver 1) has led to resistance in various bacterial strains. Increasing resistance has important economic implications as second-and third-line regimens are more expensive than first-line drugs. When first- and second-line antimicrobial treatment options are limited by resistance, physicians are forced to use antimicrobials that may be more toxic to the patient, more expensive, yet also less effective. Even when alternative treatments exist, patients with resistant infections are often much more likely to die and survivors have significantly longer hospital stays, delayed recuperation, and long-term disability [6]. More than 2.8 million antibiotic-resistant infections occur in the United States (U.S.) each year, and more than 35,000 people die as a result [7]. In addition to the toll on human life, antimicrobial-resistant infections add considerable, avoidable costs to the already overburdened healthcare system. In the U.S., studies have estimated that AMR adds $20 billion in excess direct healthcare costs, with additional costs to society for lost productivity as high as $35 billion a year [8]. The failure to act on drug-resistant infections will lead to 10 million extra deaths a year, costing the global economy $100 trillion by 2050 [9]. The loss of effective antimicrobials will also undermine the treatment of infectious complications in patients with other diseases.
Beyond AMR’s impact on human health, there is increasing concern about the enormity of the threats posed by AMR to animal and environmental health. According to the World Health Organization (WHO), in some countries, the total amount of antibiotics used in animals is four times larger than the amount used in humans [10]. Antibiotics are used for the treatment of diseases but are also widely used in animals for growth promotion and prevention of diseases. The inappropriate use of antimicrobials in livestock (driver 2) also contributes to the global challenge of AMR. Antimicrobials in livestock are used for therapeutic and non-therapeutic purposes. [7]. The development of antibiotic resistance poses a threat to the food chain since the resistant bacteria can be transmitted from animals to humans via direct contact or through the environment. The emergence of AMR among food-producing animals is a major global public health issue as some antibiotic-resistant bacteria having significant potentials to contribute to a pandemic, such as livestock-associated oxacillin/methicillin-resistant Staphylococcus aureus (ORSA/MRSA) [11][12] antibiotic-resistant Campylobacter spp. [13], and carbapenem-resistant Enterobacteriaceae or CRE [14].
Promulgation of antibiotic-resistant genes in the environment (driver 3) through horizontal gene transfer (HGT) has also been observed. HGT is acquiring foreign genes by organisms [15] (including the uptake of foreign DNA), its genetic recombination and regulatory integration, and establishment in the host population or the exchange community. The exchange community is a collection of organisms that share genes by HGT without being in physical contact. Multiple mechanisms of HGT liberate genes from normal vertical inheritance [16][17]. Studies have shown that HGT drives the evolution of untreatable “superbugs” by concentrating AMR genes together in the same cell [16].
3. AMR and Sustainable Development Goals
AMR is a complex problem with global impact and, left unchecked, it will affect the world’s sustainability and development efforts, hampering progress towards the United Nations Sustainable Development Goals (SDGs). SDGs focus on a range of interrelated goals, comprising 169 targets and 232 indicators, from the eradication of poverty and inequality to acting on climate change. Without effective antibiotics, we may fall short of many SDG targets, including SDG 1 (ending poverty), SDG2 (zero hunger), SDG 3 (global health and well-being), and SDG 8 (decent work and economic growth).
A 2019 World Bank report argues that AMR is not just a health problem, it is also a development problem [18], especially in low- and middle-income countries. AMR hits the poor and marginalized the hardest because these populations do not have access to even basic means of prevention and care, much less to effective treatments for many bacterial infections. Another World Bank report also indicated that the limited progress on containing AMR will push more than 24 million people into poverty by 2030 [2], further limiting the goal to end poverty in all its forms everywhere (SDG1).
In disenfranchised parts of the Global South (e.g., remote rural villages and city slums), confounding factors like polluting industries and insufficient access to clean water and sanitation infrastructure may exacerbate the public health problems associated with the emergence and prevalence of AMR bacteria [19]. For example, a recent review [19] unveils the link between population exposure to hazardous chemicals and the ineffectiveness of traditional WASH (water, sanitation, and hygiene) interventions in reducing diarrheal disease and child growth-stunting in Bangladesh, Kenya, and Zimbabwe. While traditional WASH strategies focus on reducing exposure to microbial pathogens, there is a failure to address the occurrence of chemical pollutants (such as pharmaceutically active compounds) in the water for human use. Thus, restricting the spread of AMR is particularly challenging in impoverished settings where vulnerability to environmental hazards is highest [20][21]. Even in urban areas that can rely on centralized waste-water treatment facilities and drinking-water plants, there have been several reports of sanitation-related outbreaks from malfunctioning and outdated water management systems [22]. Furthermore, there are limited data on the ability of water treatment technologies available in low- and middle-income countries for removing antibiotic residues and antibiotic-resistant genes (ARG).
Another region of concern is the Himalayas. Many of the Himalayan countries have a high AMR burden due to widespread use of antibiotics for therapy and of sub-optimal doses as growth promoters in animal food production, along with poor health care systems, poor infection control, and poor prevention measures [23][24]. Rural households frequently face multiple issues from extreme poverty to environmental degradation thus, attaining household nutrition security (e.g., egg production in support of maternal and child nutrition) requires a multipronged approach that is feasible in the long-term under local conditions [25]. It is a complex endeavor to meet environmental health, welfare standards, and antimicrobial stewardship at the same time. Bacterial pathogens are becoming highly resistant to most first- and some second-line antibiotics especially in poultry production systems [26]. Increased AMR in pathogens leads to increased mortality and morbidity, enhanced transmission, and increased associated health care costs [27]. For example among the BRICS nations (Brazil, Russia, India, China, and South Africa), 23% of the retail antibiotics sales were attributable to India [24][25][26][27]. The emergence of AMR is not limited to older and more frequently used classes of drugs, but also newer and more expensive drugs such as carbapenem [24]. As in many studies, children are most affected by the AMR epidemic in the Himalayas [24].
Apart from the impoverishment that comes with illness (hence, affecting SDG 3: global animal health and well-being), livelihoods that rely on food animal production, particularly livestock production, are particularly vulnerable to AMR infections. Sick workers cannot earn enough to feed their families (SDG 2: Zero Hunger), and the lingering impacts of AMR illness may impact future opportunities for employment (SDG 8: Decent Work and Economic Growth) [28].
AMR is not only critical to public health but also global development, directly impacting SDGs in the areas of poverty reduction, elimination of hunger, healthy living, economic growth, clean water, and equality. AMR also endangers recent gains in gender equality, quality education, innovation, and food production. Ignoring AMR threatens the SDG agenda for 2030, as these goals are not achievable without effective antibiotics [29]. Finding solutions to AMR is not easy. First, AMR is a concept rather than a disease that can be directly treated, yet AMR undermines the treatment of many diseases [30]. Second, AMR is global in scale, with human mobility and food trade accelerating its spread across national borders, across different bacterial species, and from bacteria in animals to those in humans [31]. Third, there is great diversity in social, economic, political, and cultural contexts in which AMR emerges or spreads [30]. Abuse or overuse of antibiotics in just a few regions is enough to overturn achievements in containing AMR elsewhere in the world [32]. Fourth, there are financial and scientific roadblocks to the development of new classes of effective antibiotics [30]. Finally, the lack of efficient and low-cost diagnostics is an obstacle to fight AMR. By not being able to tailor drug choice to infection genotype, physicians have difficulty improving treatment efficacy, which leads to inappropriate antibiotic prescription [33]. Current developments in rapid diagnostics will support the medical professionals in their on-the-spot need for information on the type of infection [34][35] but more work needs to be done. AMR strikes hardest on the poor and significantly affects women and children [36]. Thus, the spread of AMR in the low-, middle- and high-income countries endangers the continuity of sustainability efforts. Unless addressed adequately, AMR is a health, social, and economic problem that the world at large cannot afford [30].
4. Global and National AMR Initiatives
4.1. Global Health
WHO spearheaded the first global strategy focused on surveillance and research to contain AMR in 2001 [37], although the implementation of this global strategy fell short of expectations [38]. In 2015, WHO renewed its efforts on AMR and released the Global Action Plan on Antimicrobial Resistance, which was adopted at the 68th World Health Assembly in Geneva, Switzerland [39]. This was later endorsed and reinforced by the Food and Agriculture Organization (FAO) through its FAO Action Plan on AMR [40] and by the World Organization for Animal Health (OIE) through its OIE Strategy on Antimicrobial Resistance and Prudent Use of Antimicrobials [41]. While the GAP-AMR of WHO has provided an overarching framework, global strategies accordingly aligned with the GAP-AMR have also been developed to specifically address AMR in the animal health sector by FAO and OIE. Together, these three international organizations form the FAO/OIE/WHO Tripartite Collaboration on AMR to speak with one voice and take collective action to minimize the emergence and spread of AMR.
Important aspects of the recent action plans on AMR include the ability to monitor and track the different facets of antibiotic resistance and to predict AMR outbreaks. The U.S., for instance, has many methods of tracking AMR. For example, the Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network tracks healthcare-associated infection and includes information on antibiotic resistance and antibiotic use [42]. A joint program by the CDC, the U.S. Department of Agriculture, and the U.S. Food and Drug Administration (FDA), the National Antimicrobial Resistance Monitoring System for Enteric Bacteria tracks changes in the antimicrobial susceptibility of certain enteric pathogens found in ill people, retail meats, and food animals in the U.S. [42]. The CDC’s “AR Threat Reports” tracks national AMR-related deaths and infections in the U.S. [42]. CDC then submits collected data to WHO’s surveillance system, called the Global Antimicrobial Resistance Surveillance System (GLASS, launched in 2015) [43]. A component of GLASS called the Emerging Antimicrobial Resistance Reporting (GLASS-EAR) notifies the WHO when new resistance is found, which could influence how these new threats are tracked [42]. By the end of the data call in 2019, 82 countries, territories, and areas were enrolled in the AMR module. Of these, 65 provided both information on the status of their national surveillance system and AMR data for 2018 [44]. Part of GLASS is the European Surveillance of Antimicrobial Consumption Network [45].
Given the important and interdependent dimensions of AMR affecting humans, animals, and the environment, numerous countries and several international agencies have included a One Health approach within their action plans to address AMR [46][47]. The WHO describes One Health as “an approach to designing and implementing programs, policies, legislation, and research in which multiple sectors communicate and work together to achieve better public health outcomes [46]”. The GAP-AMR has spurred and catalyzed progress in addressing AMR globally, calling on the human and animal sectors of all member countries to focus on the following five (5) objectives: (1) improvement of awareness and understanding of AMR through effective communication, education and training; (2) strengthening of knowledge and evidence base through surveillance and research; (3) reducing the incidence of infection through effective sanitation, hygiene and infection prevention measures; (4) optimizing the use of antimicrobial medicines in human and animal health; and (5) development of the economic case for sustainable investment that takes account of the needs of all countries, and increase investment in new medicines, diagnostic tools, vaccines, and other interventions. The World Health Assembly urged member states to develop national action plans (NAPs) on AMR that are aligned with the overarching objectives of the GAP-AMR. Country progress in the implementation of the global action plan on antimicrobial resistance is presented in the global open access WHO, FAO, and OIE global tripartite database, which provides access to information on the status of countries regarding the implementation of the global action plan and actions to address antimicrobial resistance across all sectors.
International programs, policy initiatives, and calls for increased funding for AMR research are also changing the global climate for funding. AMR investments at national levels are still low, but recent mapping of AMR research funding indicated an increase in funding as a national priority, especially in AMR-priority areas like therapeutics [48]. Created in collaboration with WHO, the European Investment Bank, and Wellcome, the recently established AMR Action Fund, will further invest in efforts to overcome key technical and funding barriers in late-stage antibiotic development and will work in partnership with governments, multilateral organizations, and philanthropy to ensure a sustainable pipeline of new antibiotics [49].
4.2. Global Animal Health
Antibiotics are essential for combating bacterial diseases that occur in both humans and animals. However, their use in animal agriculture is increasingly scrutinized based on concerns about associations between antimicrobial usage and the development and transmission of AMR to vulnerable populations. Globally, antimicrobials are used therapeutically in animal production to treat bacterial diseases and although nontherapeutic usage (such as growth promotion) is increasingly discouraged, in some regions nontherapeutic usage is prevalent [50]. Other common uses include preventing disease during periods of increased susceptibility. For example, subtherapeutic doses of antimicrobials are commonly given to some meat-producing species (such as swine or feedlot cattle) to promote growth. AMR with its linkage to food animal production then becomes an important issue in food safety. Through DNA fingerprinting the association of drug-resistant bacteria isolated from sick people to an agricultural source has been confirmed [10]. In Asia, the presence of drug residues in the food of animal origin and antimicrobial resistance are emerging as serious problems in the region. The intensification of livestock production is accompanied by increased use and misuse of antimicrobial substances, resulting in the ineffectiveness of the drugs. Efficient and effective governance of veterinary services is a fundamental requirement for addressing the global animal health and related public health threats however, in many developing countries, veterinary services do not meet the essential requirements for good governance [51].
One barrier to reducing antimicrobial usage is the lack of standard recording systems or metrics that allow farmers to benchmark their usage. The ability to quantify antimicrobial usage on farms varies among countries, but progress on implementing a centralized international authority that tracks the use of antimicrobials in animal agriculture is being made [52]. Although progress is modest, this effort will be important in assessing the risks of antimicrobial usage on farms relative to the dissemination of resistance elements. Associations between the magnitude of antimicrobial usage on farms and the development of antimicrobial resistance are confounded by species of animal, routes of administration, class of antimicrobial, and variation in methods used to quantify usage [53][54][55][56][57][58][59][60]. Despite these challenges, consumer concerns about limiting usage of antimicrobials on farms are increasingly being voiced and policies have been enacted in several regions that have motivated food animal producers and veterinarians to further reduce antimicrobial usage [57][58][60][61].
One important practical step taken in several countries is to limit the uses of antimicrobials in animal agriculture to those classes that are less critical for human health needs [46]. Antimicrobial usage has been shown to vary considerably among farms, even those of the same species and in the same regions [57][58][59][60] indicating that achieving lower usage is feasible. Farmers who can provide better husbandry and housing for their animals typically experience fewer bacterial diseases, thus continued emphasis on extension programs to enhance animal husbandry and welfare is required. Many decisions to treat animals with antimicrobials are based solely on observation of clinical signs (rather than isolation of bacteria), so the development of inexpensive, rapid diagnostic tests to help guide treatment decisions should be prioritized. Antimicrobial usage in animal agriculture can be reduced through continued emphasis on improving animal management and providing tools to ensure judicious usage of therapeutic drugs. It is also pertinent to introduce measures for the appropriate use of veterinary biological practices and administration of drugs. The FAO recommends putting in place traceability mechanisms and other measures such as good agricultural practices (GAP), Hazard Analysis Critical Control Point (HACCP), and International Organization for Standardization (ISO) for enhancing food safety and quality as well as consumer confidence. It is also very important to promote better technologies and encourage innovation in mitigating livestock or animal diseases starting with early detection and correct diagnosis. Rapid diagnostics play an important role in preventing the misuse of antibiotics because they enable healthcare providers to select the most effective treatment for a given condition, thereby reducing the risk for antibiotic resistance and improving patient health, lowering healthcare costs, and prolonging the utility of effective antibiotics.
4.3. Diagnostics: From Biomarkers to Diseases
To control disease outbreaks, it is critical to have noninvasive diagnostic tools at low cost that provide results rapidly [62]. The first step in clinical diagnostics is to determine the causative agent of infection [63] and initial screens analyze host factors to distinguish between viral and bacterial infections. Inflammatory biomarkers such as C-reactive protein are classic signs of infection but cannot be used to distinguish between causative agents. Relatively new biomarkers such as serum procalcitonin (discovered less than 25 years ago) are becoming standards for this pre-screen [64]. The subsequent identification of the pathogen is based on a combination of biochemical, analytical, and genomic techniques. Antimicrobial susceptibility testing (AST) is used to accelerate the identification of potential therapies, and ensure suitable antibiotics are prescribed [65]. Several technologies exist for AST screening; while phenotypic characterization methods are the most common, genotypic characterization methods are emerging. Many of these emerging tools show promise for rapid quantitative analysis but require additional testing in complex clinical samples; a few key techniques are reviewed below.
Quantitative AST for Gram-positive (e.g., Staphylococcus aureus) and Gram-negative bacteria (e.g., Pseudomonas aeruginosa) are commonly available [66] but testing for pathogens such as Mycobacterium tuberculosis requires specialty laboratories. This is one example of current limitations in AMR diagnostics. New methods must be developed to expand the number of etiological agents and improve throughput without sacrificing key features like clinical sensitivity, specificity, cost, and turnaround time. Most AST turnaround times are in the 12–48-h range, but new methods are emerging to expedite the process. This speedup cannot come at the expense of clinical sensitivity or specificity, however, and systems-level analysis is sorely needed. AST may not be feasible as the sole screening tool due to the importance of species identification in establishing minimum inhibitory concentrations of antibiotics. Achievements in rapid diagnostics for pathogen and resistance factors are inspiring, but the inability to detect targets at low bacterial counts is a major problem [66][67].
To date there is no central database for comparative studies of diagnostic tools, thus technology reviews are sporadically conducted by small groups. Globally, applicable consensus standards and guidelines have been developed by EUCAST (European Committee of Antimicrobial Susceptibility Testing) and CLSI (Clinical and Laboratory Standards Institute). However, these clinical guidelines do not include technology features such as engineering metrics for guiding technology development, which creates a chasm between analytical technology research and clinical research.
4.4. Bibliometric Analysis and AMR Global Research Outputs
Mechanisms of antibiotic resistance have been a productive research topic in academia [68]. Bibliometric methods, as an adjunct to the systematic literature review, can be used to measure the importance of AMR as a research topic, provide an overview of the knowledge base, the scientific progress of AMR, and help identify its specific characteristics based on certain indicators. Bibliometrics, as defined by the OECD Glossary of Statistical Terms, is the statistical analysis of books, articles, or other publications to measure the “output” of individuals/research teams, institutions, and countries, to identify national and international networks, and to map the development of new (multi-disciplinary) fields of science and technology” [69]. As data, bibliometrics typically measures publication and citation counts, patents, royalty, and recently “altmetrics” or social media mentions. Bibliometric methods include various descriptive analyses of bibliometric data, as well as analytical methods such as statistical regressions, social network analysis (based on relationships such as co-authorships or shared citation patterns), and text mining.
Review of Bibliometrics and AMR Research
Bibliometric analysis was conducted on AMR research to validate recent bibliometric findings and support the definition and characteristics of the next generation AMR network as described here. Bibliometric data was sourced from Elsevier’s Scopus, the world’s largest abstract and citation database of peer-reviewed literature and collected in March 2021. The search phrases included “AMR”, “anti-microbial resistance”, “antimicrobial resistance”, and “antibiotic”. Searches were conducted on all indexed documents and publication types from 2011 to 2020.
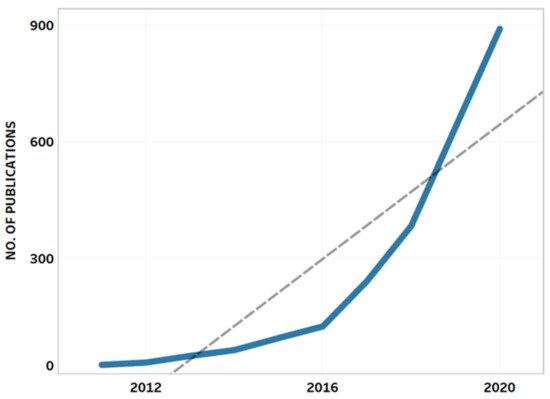
The query resulted in 2536 publications by more than 14,000 authors affiliated with more than 9200 institutions in 151 countries. The retrieved AMR publications belonged to the following subject areas: medicine (n = 1423; 31%); immunology and microbiology (n = 702; 15.3%); biochemistry, genetics, and molecular biology (n = 539; 11.7%); agricultural and biological sciences (n = 407; 8.9%); and pharmacology, toxicology, and pharmaceutics (n = 336; 7.3%). Similar to the findings of recent studies [70][71][72], growth in AMR-related scientific publications was observed over the past 10 years (Figure 1).
Figure 1. Annual growth of publications on antimicrobial resistance. The dashed line represents the trend line showing a positive trend in AMR-related research.
The growth in publication count and other bibliometric indicators (particularly the number of institutions engaged in AMR research) was obvious when the dataset was divided into two groups (2011–2015) and (2016–2020) (Table 1). A steady increase per year in the number of AMR-related publications (CAGR = 75%) and the number of contributing authors and institutions (CAGR = 81% and 86%, respectively) was noted after the release of 2015 WHO’s Global Plan of Action on AMR. The number of countries represented increased threefold, while the number of author networks increased sixfold, with a CAGR of 33% and 59% per year, respectively. Multidisciplinary research increased fivefold with a CAGR of 56%.
Table 1. Bibliometric indicators analyzed for AMR-related publications, 2011–2020.
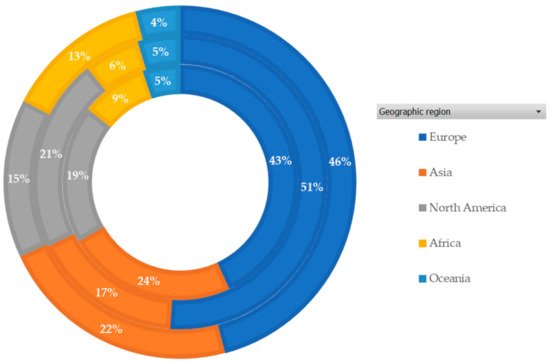
Figure 2 shows the geographic regions that contributed to the AMR research. Europe produced more than 40% of the publications, followed by Asia and North America. These regions also stand out in terms of research influence (citations) and global collaboration.
Figure 2. Percentage (%) of publications by geographic region (2011–2020). The inner circle represents the number of AMR-related publications, middle circle represents the citations received for these publications and the outer circle represents the total link strength of these regions.
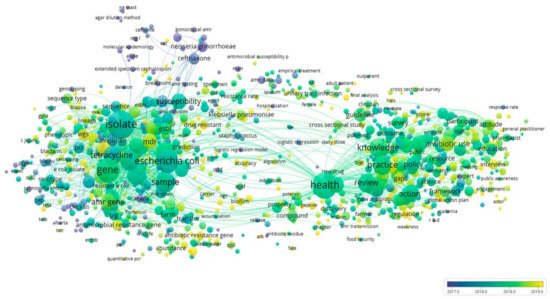
The co-occurrence of article keywords was analyzed and visualized to showcase the topical clustering and their development trajectories (Figure 3). As demonstrated in Figure 3, four major clusters were formed, with one major cluster overlapped with the others. The clusters presented in green represent the research on antibiotic consumption and stewardship programs about AMR and overlapped with the others. The clusters in yellow represent the AMR management in health, agriculture, and environment sectors while the clusters in blue represent AMR surveillance, infection control, and risk assessment from a medical perspective. Finally, the clusters in purple represent AMR causes and testing, and first line of treatment. Figure 3 reveals that in recent years, research topics have developed from AMR causes, testing and initial treatment, to AMR surveillance, infection control, and risk assessment, to antibiotic consumption and stewardship programs, and to most recently, the push for a One Health approach including management of AMR in animal and environmental sectors.
Figure 3. Co-occurrence network of keywords. The node size indicates the number of occurrences of a keyword. Edges between nodes indicate co-occurrences between keywords. The thicker an edge is, the more a keyword co-occurs with the other keyword. The node color indicates the category of a node and is scaled to the averaged publication year.
Interestingly, keywords indicating environmental research were predominantly clustered with keywords relating to agriculture and food production suggesting the need for an integrative perspective uniting agriculture, food, and the environment, hence, a one health approach. However, despite this grouping, keywords relating to One Health research in the current dataset were relatively isolated as they demonstrated less dense connections with other keywords or clusters. This implies that the One Health approach remains at the periphery of the AMR research but its location in the network is expected to improve with greater focus on this approach to better understand drivers of the global emergence, dissemination and curb the effects of AMR.
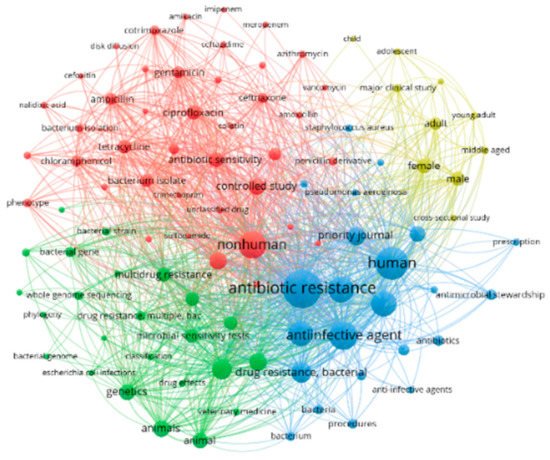
Mapping the top 100 most frequently used terms in the retrieved AMR-related publications resulted in four major clusters around different research themes (Figure 4). The first research theme (red cluster, n = 41 terms) represents research on new drugs and antibiotics to treat infections and control AMR. The second theme (green cluster, n = 24 terms) represents research on antibiotic resistance in bacteria from humans and farm animals. The third research theme (blue cluster, n = 23 terms) represents antibiotic stewardship and measures for optimizing the prescription of anti-infective agents. The fourth research theme (yellow cluster, n = 10 items) relates to knowledge, practices, and attitude toward antibiotics use. These clusters along with other research themes are complementary to the research areas that the next generation of AMR network provides in this entry.
Figure 4. Network visualization map of top 100 most frequently used terms in the retrieved AMR-related publications (2011–2020).
The growth trends of the last decade are expected to continue as scientific research on AMR increases in urgency. To foster this growth, it is critical to build research networks and capacity for transdisciplinary and multidisciplinary research on AMR. The next section defines the next generation of AMR networks, including proposed hallmarks, phase-gate milestones, and strategies to fill AMR implementation gaps.
5. Defining the Next Generation of AMR Network
A global network-to-network (NTN) collaboration is needed to accelerate the process of scientific discovery, identify effective AMR solutions, and integrate them into existing systems. This next-generation AMR network shall also prepare current and future researchers for the type of multi-team, interdisciplinary, global collaborations required to address AMR.
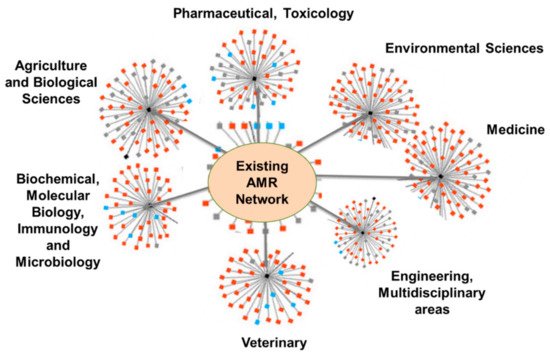
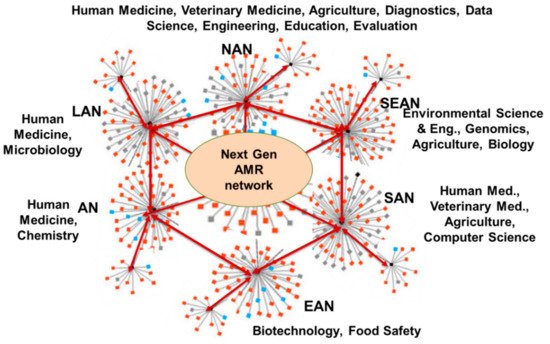
Members of this NTN shall represent economies, locations, climates, cultures, and resources across multiple continents, and should be a collegial group of members from lower- and middle-income countries (LMICs) and high-income countries (HICs). Existing individual, institutional, national, regional, and global networks (Figure 5) that are discipline-based will feed into this proposed AMR NTN system and develop the transdisciplinary research roadmap that will foster innovation and discovery for addressing AMR. Figure 6 illustrates how the transdisciplinary, next-generation AMR network will leverage regional and disciplinary expertise within the nodes and develop new connections between regional networks.
Figure 5. Existing research network contributing to tackling AMR (Network images were adopted from Google Images.).
Figure 6. Next-generation AMR network. NAN = North American Network; LAN = Latin American Network; SEAN = Southeast Asian Network; SAN = South Asian Network; EAN = East Asian Network; AN = African Network.
Many multi-institutional scientific networks are already contributing to the fight against AMR, as evidenced by available literature and bibliometric analysis. Sustaining these networks will be very important as we develop the AMR NTN. Other, emerging research networks are contributing also to AMR control by developing much-needed rapid diagnostics for quickly discriminating bacterial from viral infection. One such network management is the Global Alliance for Rapid Diagnostics (GARD) (https://www.egr.msu.edu/alocilja/GARD-location/global-alliance-rapid-diagnostics-gard, accessed 5 May 2021), which was formed in 2016 as a peer-to-peer collaboration of equals representing scientists and practitioners from almost all disciplines where AMR is a focus. GARD’s team has members from countries spanning five continents. GARD’s mission is to develop portable, affordable, globally deployable, nanotechnology-enabled biosensing technologies for rapid and early detection of infectious diseases. This effort is focused particularly on serving populations who are most in need of these technologies but least able to afford them.
GARD’s efforts have led to the formation of six regional networks representing North America, Latin America, Southeast Asia, South Asia, East Asia, and Africa. These GARD members are working together to develop state-of-the-art, rapid biosensing and diagnostic technologies that are broadly accessible due to their low cost, simple operation, and room temperature storage. GARD members are also working to develop biosensing assays for drug-resistance profiling of infectious diseases, and on developing smartphone-based data analytics and artificial intelligence (AI) algorithms. These apps will support medical and veterinary professionals in prescribing antimicrobials in prudent and responsible ways. The collaboration has so far resulted in jointly authored journal publications; faculty and student exchanges; jointly sponsored conferences; and training a new generation of scientists in the multidisciplinary and geographically specific approaches to AMR problem-solving. GARD can play an important role in pursuing the mission and vision of the next generation AMR network.
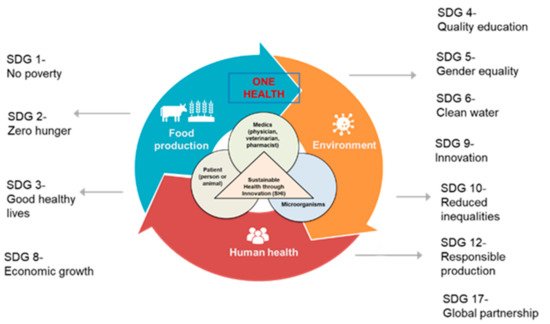
The new generation of AMR network can focus on promoting a new, transdisciplinary paradigm called “Sustainable Health through Innovation (SHI)” (Figure 7). SHI will transcend traditional disciplinary boundaries to develop AMR solutions guided by the One Health approach in the context of SDGs and find ways to use technological innovations (diagnostics including antimicrobial susceptibility tests and decision support tools) to bring about long-term solutions. SHI is expected to contribute to 11 of the 17 SDGs in the 2030 plan. The SHI paradigm can be achieved by (1) developing an intentional, transdisciplinary research roadmap; (2) designing and implementing sustainable digital and data-based solutions that promote interdisciplinarity, real-time insights, and decision making; and (3) implementing effective and impactful capacity building and training programs designed to produce a new class of transdisciplinary scientists and researchers contributing to tackling AMR globally. The end goal includes the prudent use of antimicrobial medicines, slowing down the emergence of AMR, prolonging the lifespan of existing antimicrobials, conserving healthcare resources, limiting the adverse economic impact of AMR, building best practices for the rational use of antimicrobials, and improving clinical outcomes for the global population.
Figure 7. The SHI paradigm supporting the next generation of AMR network and its impact on 11 out of 17 SDGs (Some images were adopted from Google Images.).
5.1. Antimicrobial Stewardship
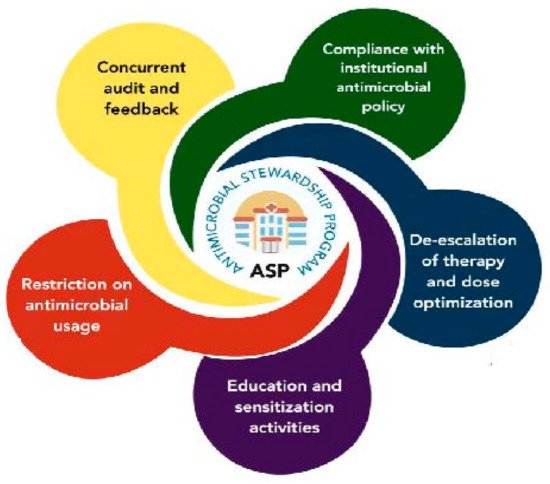
Establishing and implementing needs-driven, evidence-based antimicrobial stewardship programs (ASP) is essential to the AMR research roadmap, as illustrated in Figure 8 for example. The ASP would be characterized by compliance with institutional antimicrobial policies with concurrent audit and feedback for the purpose of restricting antimicrobial usage and the de-escalation of therapy through use of optimized doses. Education is a major component of the whole ASP program. The expected outcome of an effective ASP are appropriate usage (choice, dose, route, and duration) of antimicrobials, less prophylactic therapy, and more empirical basis of prescriptions [73], In 2019, WHO released an ASP toolkit to guide healthcare facilities in low- and middle-income countries [74]. The toolkit provides general guidance for antimicrobial stewardship team structures, resources, and competencies that can help change antibiotic prescribing practices. Although there is scientific evidence on the benefits of ASP implementation [73] and national, regional, and global guidance documents exist, there is a growing need for more specific guidance on how to establish, implement and evaluate effective ASPs at the healthcare facility level, especially in less developed nations [73]. These stewardship programs shall also focus on the impact of the speed of diagnosis; availability of drug susceptibility testing; pathogen-specific margins between expectations of spontaneous cure and treatment cure; state of bacterial infection on antimicrobial prescribing; timely and appropriate dosage, initiation, administration, and de-escalation of antimicrobials; and clinical outcomes of infection.
Figure 8. Illustration of an Antimicrobial Stewardship Program (ASP) framework.
There are also stewardship practices and enabling technologies within and across existing AMR networks. These existing practices and technologies, including factors that affect their implementation, must be mapped, and shared responsibly. A key source is institutional data, especially from hospitals and clinics, including antimicrobial consumption and/or use, prescription audits, and AMR surveillance data. Building on existing AMR stewardship competencies is important to identify (1) the characteristics and requirements of a sustainable ASP governance structure; (2) the immediate and long-term priority areas and corresponding resources; (3) the parameters and action items to strengthen knowledge sharing, collaboration and networking supporting the SHI paradigm; (4) the technologies and solutions for impactful results; and (5) the challenges and opportunities for a team-based approach.
In addition to physicians, patients and pharmacists can play major roles in AMR reduction and antimicrobial stewardship approaches [75][76]. Improved knowledge of the AMR problem from the perspective and collaborative experience of physicians, veterinarians, and pharmacists is fundamental for controlling antimicrobial use. Partnerships between doctors and pharmacists are increasingly enabled by mobile decision support applications. These apps connect to databases containing information on (1) diseases and corresponding microbial agents; (2) antimicrobial treatment options; (3) susceptibility of the disease agents to available antimicrobials; (4) drug resistance emerging in hospitals, communities, animal production, and the environment in the vicinity; and (5) commercially available antimicrobials.
Antimicrobial resistant organisms (AROs) in food-producing animals and organisms that can cause disease in humans include enterococci, Escherichia coli, Campylobacter, and Salmonellae [77]. Antimicrobial stewardship in food production has been associated with decreased rates of resistance in both animals and humans-without reducing farm productivity [78]. Improved surveillance, stewardship, and advocacy are needed to prevent AROs from entering the food chain. Strategies include the collection of annual surveillance data on antibiotic resistance in humans, animals, retail meat, and environmental samples; using non-antibiotic prevention approaches; establishing certification standards for antimicrobial stewardship; lobbying hospitals and health care institutions to source meat raised without antibiotics; and collaborating with advocacy groups to promote One Health stewardship [66]. Antimicrobial Stewardship program (ASP) guidelines have been developed for high-income countries’ health systems where there are adequate personnel, laboratories, and supplies. However, most health systems across the globe lack the resources to establish sustainable stewardship programs, which is concerning because these lower-resourced systems are the main source of antimicrobial misuse and AMR spread. Additional resources and training are required for the new stewardship guidelines to make a difference in AMR in lower- and middle-income countries.
The need is tremendous: there are no antimicrobials available to treat moderately drug-resistant (MDR), extensively drug-resistant (XDR), or Pan-resistant (superbug) infections. Broad-spectrum antimicrobial combinations are the last resource to treat pan-resistant bacteria, although they are not always effective. AMR due to these “superbugs” is seen in hospitals around the globe with increasing frequency, and a lack of proper equipment and specialized personnel in low- and middle-income countries means that pan-resistant infections may be underreported. The emergence of new bacterial inherited (plasmids/genes) or acquired resistance mechanisms further threatens human health. The current “ad intra” hospital-centralized model to prevent, fight, and contain AMR spreading is no longer sufficient. Instead, we need a new, more proactive, “ad extra” paradigm to search for new sources of AMR in every context where antimicrobials are used (human, animal, and environmental health). This new paradigm will rely on cost-effective technologies that are easy to use at the point of care to isolate, detect, characterize and phenotype bacteria, with the results made readily accessible to inform local treatment decisions.
Numerous studies combined the detection of antibiotic-resistant genes (ARGs) in the environment (e.g., water) with the estimation of species composition through metagenomic analysis (e.g., 16S rRNA metabarcoding) [79][80][81][82][83][84]. The detection of ARGs in environmental bacteria has been based solely on isolated and cultured bacterial species, which does not identify the percentage of unculturable bacterial species that may possess certain ARGs. This has been the major challenge in AMR/ARG research, most notably those incorporating next-generation sequencing (NGS) technology. There is a lack of comprehensive studies investigating the dynamic aspects of ARGs in the aquatic environment, due to technological or methodological constraints. With the advent of newer molecular-based technologies, whole-genome sequencing (WGS) and whole-metagenome sequencing (WMS) analyses are now achievable for studying bacterial species or communities, respectively [85][86][87]. MinION Oxford Nanopore [88][89] and Novaseq (Illumina) [90] are prime examples of this technology. Such analysis in bacterial communities can elucidate the status of ARGs in a comprehensive range of species, including uncultivable bacterial species. It can also reveal the presence or absence of all mobile genetic factors (horizontal gene transfer of antimicrobial resistance genes among bacterial species in the environment) distributed in the genome of each bacterial species in the community. Conducting WGS in bacterial communities can potentially reveal which species in the community possess specific antimicrobial resistance genes. Furthermore, it is a culture-independent technique, which is not technically difficult, costly, or time-consuming as compared to conventional methods. Thus, WGS is deemed as an effective tool for predicting and controlling ARGs in both clinical and environmental settings.
5.2. Data-Based Tools and Mobile Applications
The growing interactions between field data, algorithms, big data analytics, connected things, and people offer new opportunities to manage AMR. Careful and strategic use of digital technologies and data can support digital transformation across all sectors of economies and societies and have wide effects in mitigating the effects of AMR. The increasing availability of medical data and artificial intelligence offers opportunities for instant AMR diagnosis, and possibilities for scientific discoveries, new products, and services for controlling AMR [91][92][93]. These benefits can be realized at local and regional levels by creating an inventory of existing data sources and features, including open access and accessibility, and mapping important fields and variables that can be used to integrate information from multiple sources in a meaningful and secure manner.
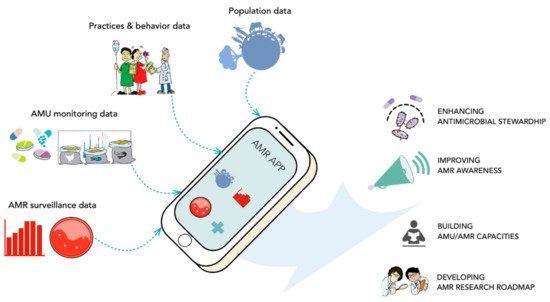
This approach could lead to the design and scaling of a decision-support mobile application and digital platform that can help address AMR-related issues at the patient, community, and ecosystem levels. These tools will also increase understanding of how access to safe antimicrobials and rapid diagnostics influence AMR. Information from national and global epidemiological surveillance, such as GLASS, can be incorporated into these tools for diagnostics and prevention. The tools can also be used for education, increasing awareness of AMR, and explaining user-implemented solutions. Examples of this type of user-level education on infection prevention and control might include explanations of the importance of taking all prescribed doses of treatment and only using drugs that were prescribed to the patient; the detrimental effects of using substandard/counterfeit medication; and the usefulness of hand washing. These tools can also be used by prescribers in real-time to seek guidance about the most effective and prudent drug prescription for each unique case, based on pathology and local/regional AMR concerns. Figure 9 illustrates this decision-support mobile app, its requirements, and features.
Figure 9. A sample schematic of the mobile-operated decision-support tool for antimicrobial stewardship and resistance control. (Images were adopted from Google Images.).
5.3. Training and Network Linkages
The next generation AMR network is a unique opportunity for the convergence of groups that differ in disciplinary expertise, geography, and culture, but are united to address the global problem of AMR. This convergence can only happen with intentional professional development training, research exchanges, and knowledge-sharing activities that build capacity in the global workforce to address the challenge of AMR. The next generation AMR network described here includes professional skills training designed to build capacity in communications, teamwork, and leadership in the context of multidisciplinary, collaborative research. Based on the CyberAmbassadors program [94] funded by the National Science Foundation, this training is modular and highly customizable and includes a “train-the-trainers” component that prepares local facilitators to provide training on a regular basis, allowing for rapid scaling and deployment of professional development on a global scale.
6. Conclusions and Prospects
The overarching vision of the next generation AMR network is to reduce the spread of antimicrobial resistance through convergent antimicrobial stewardship approaches guided by the Sustainable Health Innovation and One Health (human health, food production, and environment) paradigms, in the context of Sustainable Development Goals. Converging global efforts to improve antimicrobial stewardship through science- and evidence-based knowledge and solutions can help reduce the spread of AMR and prolong the usefulness of antimicrobials. The expected impact from this AMR network is enormous: (1) convergent antimicrobial stewardship approaches supported by modern tools to prevent AMR-related mortality and morbidity; (2) a strengthened educational and research enterprise that fosters a globally minded and culturally diverse workforce tackling AMR; and (3) an active international network and dynamic database of AMR experts that could be tapped quickly in case of global pandemics.