Cilia are comprised of microtubule bundles organised into an axoneme and anchored by a mature centriole or basal body. Primary cilia are dynamic signalling platforms that are intimately involved in cellular responses to their extracellular milieu. Cilia on fibroblasts/fibro–adipogenic progenitors and myofibroblasts may influence cell fate in both a cell autonomous and non-autonomous manner with critical consequences for skeletal muscle ageing and repair in response to injury and disease.
- myogenesis
- primary cilia
- proliferation
- differentiation
- satellite cells
- cytoskeleton
- extracellular matrix
1. Introduction
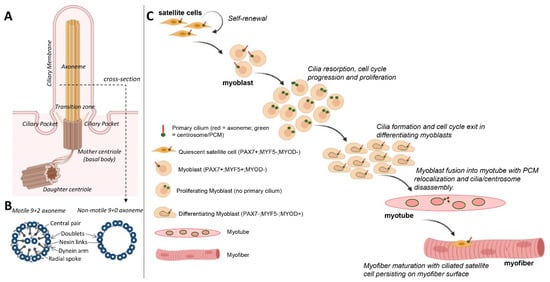
2. Formation and Resorption of Cilia during Skeletal Myogenesis
In skeletal muscle tissue, cilia are found on mononucleated cells that include satellite cells/myoblasts and resident fibro/adipogenic progenitors in the interstitial connective tissue [10][11][10,11]. The presence of cilia on myoblasts was described 50 years ago in ultrastructural studies for avian and then human myoblasts in culture [12][13][12,13] and in regenerating adult mouse muscle [14].
In primary myoblast cultures, following serum withdrawal, ciliogenesis coincides with cell cycle exit and the early onset of differentiation (Figure 1C) [15]. This is consistent with the notion that the formation of cilia is an anathema to cell division. For example, cilia disassembly is required prior to mitosis, as the mother centriole that comprises the basal body of cilia is required for spindle assembly [16]. Moreover, mitotic kinases required for spindle assembly, such as Aurora A, conversely promote the disassembly of cilia [17]. With the progression of differentiation and prior to myoblast fusion, cilia on myoblasts are disassembled and are largely absent in mature, multinucleated myofibers (Figure 1C) [15]. Coincidentally, it is also during this time that myoblasts undertake a process of centrosome reduction to facilitate a switch to non-centrosomal microtubule organisation [18]. Prior to fusion into multi-nucleated myotubes, proteins that seed and anchor microtubules, such as γ-tubulin, pericentrin and ninein, relocalise from the pericentriolar matrix to nuclear and Golgi membranes, centriole pairs split due to the cleavage of protein tethers and centrosomes are reduced in size and disassembled (Figure 2). The reduction in centrosomes would be expected to trigger cilia disassembly, but whether the two processes are linked and how they contribute to myogenesis are not well understood.
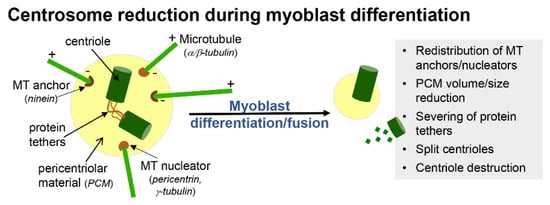
One possibility is that ciliogenesis may help sequester a proportion of myoblasts in a quiescent state but with a retained proliferative capacity, while the reduction in centrosomes is associated with a transition to a terminally differentiated state for myotube/myofiber formation. In support of this, quiescent satellite cells in vivo or in ex vivo myofiber explants retain a primary cilium, and this is disassembled as satellite cells re-enter the cell cycle to divide and regenerate muscle in response to experimental cardiotoxin-induced myofiber necrosis [10]. Cilia are briefly reassembled in differentiating MyoD+ve myoblasts but are absent in myofibers (Figure 1C), consistent with their disassembly prior to or shortly following myoblast fusion [10].
3. Cilia and Cell Cycle Regulation in Myoblasts
Ciliogenesis is intimately coupled with the cell cycle. The primary cilium is enriched with G-protein-coupled receptors, receptor tyrosine kinases, chemo- or mechanosensing receptors and downstream signal transduction proteins that relay external cues to cell cycle regulators to ultimately determine cell proliferation, self-renewal and cell fate [19]. Moreover, ciliary trafficking, mediated by intraflagellar transport (IFT) protein complexes, has reported functions in cell cycle regulation [20][21][20,21]. Thus, abnormalities in ciliogenesis or cilia-mediated signal transduction often lead to cell cycle dysfunctions that are associated with ciliopathies and tumourigenesis [22][23][22,23]. However, the molecular relationship between cilia and the cell cycle is complex and may be contextual, with cell type-specific differences.
Typically, in dividing vertebrate cells, cilia are assembled during G0/G1 and disassembled prior to mitotic entry [23]. Within stem and progenitor populations, the formation of cilia is associated with reversible quiescence, and their disassembly is thought to be tied to cell cycle re-entry and the reinitiation of division for restorative purposes [6]. In most cells, cilia persist through the duration of G1 and are disassembled at G1-S transition through Aurora A kinase (AURKA)-mediated resorption of the ciliary axoneme [24], and live imaging studies of cultured fibroblasts revealed that cilia resorption was preceded by the extracellular release of a ciliary vesicle from the distal tip, a process termed ‘ciliary decapitation’ [25].
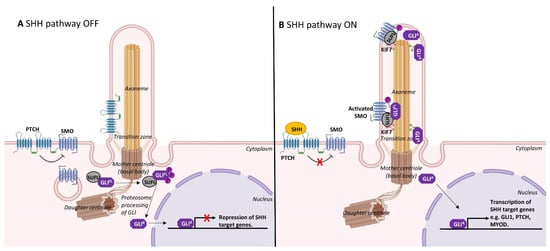
The dynamic assembly/disassembly of cilia during skeletal myogenesis is consistent with a role in restraining cell proliferation. As mentioned above, cilia are prominently observed on quiescent satellite cells or immediately following cell cycle arrest as myoblasts differentiate [10][15][10,15]. The formation of cilia is involved in the spatiotemporal co-ordination of the Hedgehog signalling pathway, which is critically required for skeletal muscle morphogenesis [31]. In mammals, Sonic hedgehog (SHH) is the most well studied of the Hedgehog family of secreted signalling proteins and binds its cognate receptor, Patched1, a cell-surface transmembrane protein that is localised to cilia (Figure 3). In the absence of SHH, Patched1 prevents the accumulation of a G-protein-coupled receptor, Smoothened, within the primary cilium to maintain inactive Hedgehog signalling [32]. SHH binding to Patched1 leads to the accumulation and elevated activity of Smoothened, which in turn activates Gli family transcription factors to regulate myogenesis (Figure 3) [33].
4. Cilia Maintenance and Satellite Cell Fate
Some studies suggest that primary cilia and associated Notch and Hedgehog signalling may be involved in determining cell fate during skeletal myogenesis [10][37][38][39][10,37,38,39].
Primary cilia were predominantly detected in Pax7+ve satellite cells but not in activated (Myf5+ve) and differentiating (Myogenin+ve) cells in normal adult skeletal muscle (Figure 1C). The chemical inhibition of primary cilium formation in isolated myofibers led to reduced numbers of Pax7+ve cells and increased numbers of Myogenin+ve cells, which suggests that cilia are required to maintain muscle stem cells [10]. Moreover, primary cilia were observed on proliferating transit-amplifying myoblasts undergoing symmetric cell division. However, during asymmetric division of satellite cells, the primary cilia are also distributed asymmetrically and associated with the self-renewed Pax7+ve daughter cells [10]. Moreover, a study has shown that the ciliary membrane attached to the mother centriole was endocytosed at the onset of mitosis, localised to one centrosome during mitosis and was asymmetrically inherited by one daughter cell. This daughter cell was able to reassemble a functional primary cilium and retained stem cell character compared to the non-inheriting daughter cell [40]. Thus, primary cilia appear to be asymmetrically inherited by a pool of undifferentiated satellite cells and may contribute to their capacity to self-renew.
Ciliary Hedgehog signalling may act as a gatekeeper in determining cell fate [41][42][41,42]. Indeed, the transcription factor Gli3 is processed into its repressor form at the primary cilium (Figure 3), and this appears to keep satellite cells in the quiescent state. Gli3-depleted satellite cells were rapidly activated, entered the cell cycle and underwent symmetrical cell division in the absence of injury or Hedgehog stimulation [43].
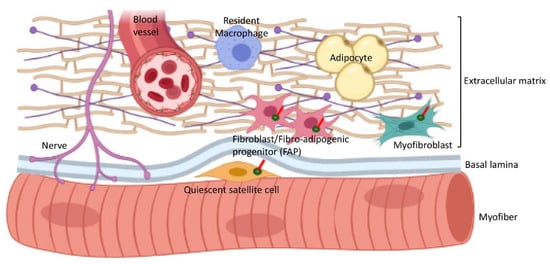
In addition to satellite cells, other populations of cells in the interstitial connective tissue of skeletal muscles are also ciliated (Figure 4, Table 1). These include mesenchymal stem cells and fibro–adipogenic progenitors (FAPs) that are considered to be very similar or identical to fibroblasts [48] and can differentiate into myofibroblasts or adipocytes [49][50][51][49,50,51].
| Cell Type | Targeting | Cellular Phenotype and Signalling Consequences | Reference |
|---|
| C2C12 and primary murine myoblasts | miRNA silencing of CEP290, IFT80 or IFT88 Ciliobrevin D |
Increased proliferation. Reduced differentiation and MRF (MyoD, Myf5) expression. Reduced myoblast fusion and myogenesis. Inhibited Hedgehog signalling. |
[15] | |||
| C2C12 myoblasts | siRNA silencing of IFT88 | Altered quiescence. Reduced self-renewal potential. Enhanced progression to G2/M. Enhanced growth factor signalling to mTOR. |
[29] | |||
| Pax7 | +ve | satellite cells within isolated myofibers | Nocodazole or Taxol. Forchlorfenuron (septin inhibitor) |
Reduced self-renewal. Increased myogenin expression. |
[10] | |
| Fibro–adipogenic progenitors (in vivo) | Pdgfra | -CreERT deletion of IFT88. | Decreased differentiation to adipocytes and reduced adipogenesis. Increased myofiber size in | Dmdmdx | and following injury. Derepressed Hh target genes (Gli1 and Ptch1). Enhanced TIMP3 expression. |
[5] |
| Adipose progenitors (in vitro) |
siRNA silencing of Kif3A. Ciliobrevin D |
Reduced differentiation to myofibroblasts. Inhibited TGFβ signalling. |
[30] | |||
| Myofibroblasts (in vitro) | Ciliobrevin D | Decrease in myofibroblast phenotype. | [30] |
5. Cilia and Myoblast Fusion
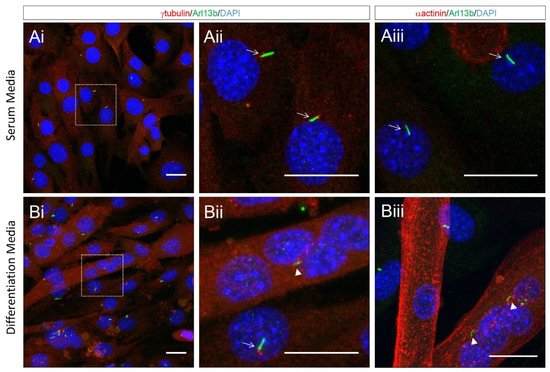
The disassembly of cilia during myoblast fusion suggests that ciliary functions may not be required for this stage of myogenesis. However, our close inspection of differentiating cultured myoblasts indicates that remnants of the ciliary membrane, as marked by Arl13b, attached to centrioles are clearly visible within multi-nucleated myotubes (Figure 5). Moreover, we observed multiple internalised ciliary remnants within multinucleated myotubes (Figure 5). This indicates that ciliary structures are not completely disassembled and are retained for some time in newly formed myofibers. This is analogous to the retention and internalisation of ciliary membrane remnants during cell division in cultured epithelial cells and radial glia progenitor cells in the developing forebrain [40]. As cilia remnants are specifically inherited by a single daughter, this has the potential for asymmetric signalling and fate consequences between the progeny of cellular mitoses [70]. The functions of remnant cilia are generally underappreciated, and it remains unknown if these structures continue to signal following internalisation. One could speculate that the internalised remnant of primary cilia following myoblast fusion may contribute to the normal development and maturation of newly formed myofibers.
6. Cilia Interactions with the Extracellular Matrix (ECM) in Skeletal Muscle
In the context of other tissues, there is abundant evidence that cilia mediate cellular responses to the ECM and, vice versa, the remodelling of the ECM by ciliated cells [72][73][72,73].
The excessive fibrosis and pathological accumulation of ECM proteins observed in ‘ciliopathies’, such as renal cystic and Bardet-Biedl syndromes, suggests a proper cilia–ECM dialog is important for normal cellular functions and organ development. The ECM influences cilia length and ECM components, such as laminin, and collagen has been shown to promote cilia growth [74][75][74,75]. There is increasing evidence that cilia signalling influences cellular adhesions and communication with the basement membrane. In addition to receptors that respond to various growth factors and morphogens in the extracellular space, the ciliary membrane, in chondrocytes and osteoblasts, for example, are also comprised of integrin receptors and chondroitin sulphate proteoglycans that respond to ECM proteins for mechanotransduction and proper bone development [76]. Cilia in osteocytes contact collagen fibres in tendons and epithelial cells in hair follicles, and developing mammary glands are oriented towards the ECM and are involved in normal development [77][78][77,78]. Ciliated satellite cells in skeletal muscle may similarly ‘scan’ the ECM, and this may influence cellular decisions to proliferate, differentiate or return to quiescence. Collagen plays a crucial role in regulating satellite cell self-renewal [79], and the loss of fibronectin and integrin signalling impairs muscle regeneration [80]. Likewise, other ECM components, such as TGF-β, glycosaminoglycans and hyaluronan, have been shown to inhibit myoblast differentiation and subsequent fusion and myotube formation [71][81][71,81]. The ECM in aged skeletal muscle also alters satellite cell differentiation in response to injury, triggering their conversion to fibroblasts to exaggerate fibrosis [82]. Thus, it is likely that the mechano-, chemo-sensory functions of cilia would contribute to ECM influences on satellite cell behaviour and myogenesis, although this has yet to be specifically tested. It is well established that satellite cells respond to ECM remodelling during muscle adaptations to ageing and to damage and inflammation during muscular dystrophies [71].
In addition to sensing the extracellular environment, ciliary signalling in satellite cells and differentiated myoblasts may also contribute to remodelling of the ECM. Hedgehog signalling in chondrocytes brings about changes in ECM composition associated with bone development [83].
Feedback mechanisms between cilia and the ECM may act in a cell autonomously or non-autonomously manner in satellite cells and FAPs/fibroblasts resident in connective tissue as important mediators of muscle growth, repair, homeostasis and ageing. They also contribute myoblasts to muscle regeneration in response to myonecrosis resulting from either intrinsic muscle degeneration (as in DMD) or experimental or accidental injury.
