Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Craig Vierra.
Spider silk is a high-performance fiber renowned for its extraordinary mechanical properties, rivaling steel, Kevlar, and a wide range of other natural and manmade materials.
- spider silk
- spidroins
- tubuliform silk
- black widow spider
1. Introduction
Spider silk is a high-performance fiber renowned for its extraordinary mechanical properties, rivaling steel, Kevlar, and a wide range of other natural and manmade materials [1,2][1][2]. The western black widow, Latrodectus hesperus, has become one of the most extensively studied spider species with respect to silk gene expression and fiber constituents, both at the level of transcriptomics and proteomics [3,4,5,6,7,8,9,10,11][3][4][5][6][7][8][9][10][11]. Black widow spiders, which are cobweavers, are members of the superfamily Araneoidea. Morphological studies of female black widow spiders reveal seven distinct silk-producing abdominal glands, including the major ampullate, minor ampullate, tubuliform, aciniform, pyriform, aggregate, and flagelliform glands [12,13][12][13]. Each gland produces fibers or glues with distinct mechanical and biomolecular properties that are highly tuned to specific biological functions [14]. Differences in the mechanical properties of the threads are largely due to altered protein compositions, which are strongly correlated with differential expression of structural proteins called spidroins, a contraction of the words spider and fibroin [15,16][15][16]. Spidroins, therefore, represent a family of spider-specific fibroins that have significant roles in fiber mechanics. Members of this family have common biochemical characteristics, including large molecular masses with internal block repeats and non-repetitive N- and C-terminal domains within their protein sequences [16,17,18,19][16][17][18][19]. Although the spidroin family protein architectures all share internal block repeats, their specific amino acid sequences and lengths differ, and these differences in primary amino acid sequences have been a focal point of the scientific community to establish relationships between protein sequence, structure, and fiber mechanics [20,21,22][20][21][22].
The major ampullate and tubuliform glands have different morphological features, but both lumens store a liquid spinning dope that is extruded into a dry fiber via external spigots on the spider’s spinnerets. Despite morphological distinctions, these two glands are reported to share similar spidroin gene expression profiles [3]. In black widow spiders, the major ampullate gland extrudes dragline silk (also called major ampullate silk or MA silk), a fiber type used for locomotion and web construction, whereas the tubuliform gland manufactures the main constituent of egg cases, tubuliform silk. Egg cases, which also contain aciniform silk, enclose eggs and protect them from predators and environmental fluctuations, such as changes in temperature and humidity [23]. Tubuliform silk has been shown to contain the spidroin TuSp1, along with two non-spidroin proteins called egg case protein 1 and 2 (ECP1 and ECP2) [6,24,25,26][6][24][25][26]. Additionally, a recent proteomic analysis of tubuliform glands, along with deep transcriptome sequencing studies to identify silk-gland specific transcripts (SSTs), has led to the discovery of 14 SST proteins in tubuliform glands of black widow spiders [5]. Two of these 14 SST proteins have been previously shown to be constituents of tubuliform silk, which include ECP1 and TuSp1 [6,24,25,26][6][24][25][26]. However, it is unclear whether the other 12 SST proteins are extruded from the tubuliform gland, or alternatively, whether these factors represent non-extruded proteins that participate in the spider silk assembly pathway. To further emphasize the importance of this work, the identification of novel constituents of spider silk, specifically tubuliform silk, has significant value for the spider silk community, as insight into the ingredients that comprise natural silk has direct implications on synthetic silk production and its potential use for military, industrial, and engineering applications.
2. Proteomic Analysis of Tubuliform Glands, Spinning Dope, and Egg Sacs
In order to perform a comprehensive, in-depth analysis of the constituents of tubuliform silk, we developed a generalized proteomic workflow designed to analyze proteins expressed in tubuliform glands, the spinning dope, which represents the liquid components within the lumen of the glands and the egg sacs of black widow spiders using mass spectrometry (Figure 1). Scanning electron microscope studies of black widow spider egg cases have previously revealed two distinct fiber types [25]. The diameter sizes are approximately 5 μm and 0.5 μm, corresponding to tubuliform and aciniform silk, respectively, with the majority of the volume comprising tubuliform silk (Figure 2A,B).
Figure 1. General workflow for isolation of the tubuliform gland, egg sac, and dope (liquid removed from the tubuliform glands), followed by analysis of the tryptic peptides by MS/MS analysis.
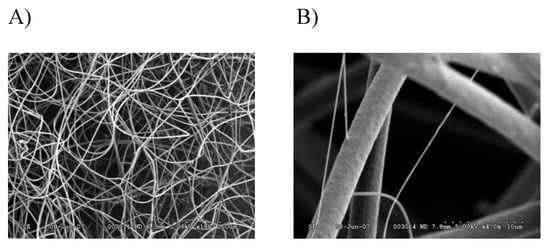
Figure 2. SEM images of black widow spider egg case silk reveal two different diameter fibers: (A) 180× magnification; (B) 4000× magnification.
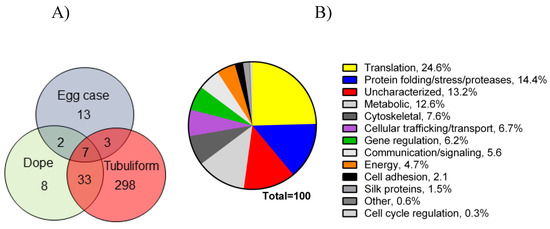
Figure 3. Venn diagram representing number of proteins expressed in tubuliform glands, liquid spinning dope, and egg sacs, along with a pie chart of percentages of proteins expressed in the tubuliform gland categorized into several different molecular and cellular functions: (A) Venn diagram; (B) pie chart.
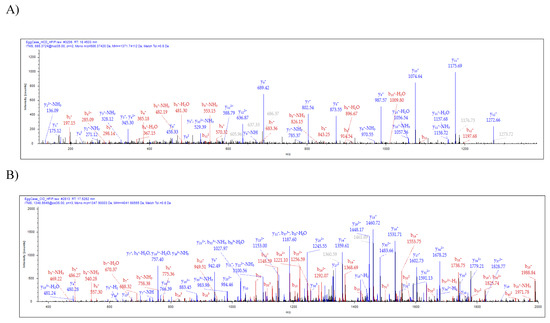
Figure 4. Tandem MS analysis of tryptic fragments generated from in-solution digestion of tubuliform spinning dope identifies the presence of a CRP4 peptide sequence, VVGPFPICDYGLR, and peptide sequence of an uncharacterized protein, SQGNVAMSSSQAGYG QGQSYSSNYAATGDSGTGQGGYSSMR. (A) HCD (MS/MS) spectrum of CRP4 precursor ion with MH+ m/z 1271.74 supports the presence of CRP4 in spinning dope; (B) CID (MS/MS) spectrum of the uncharacterized protein’s precursor ion with MH+ m/z 4041.68 supports the presence of ECP3 in spinning dope. Y- and b-ions and their corresponding masses are shown above the peaks.
References
- Gosline, J.M.; Guerette, P.A.; Ortlepp, C.S.; Savage, K.N. The mechanical design of spider silks: From fibroin sequence to mechanical function. J. Exp. Biol. 1999, 202, 3295–3303.
- Moore, A.M.; Tran, K. Material properties of cobweb silk from the black widow spider Latrodectus hesperus. Int. J. Biol. Macromol. 1999, 24, 277–282.
- Lane, A.K.; Hayashi, C.Y.; Whitworth, G.B.; Ayoub, N.A. Complex gene expression in the dragline silk producing glands of the western black widow (Latrodectus hesperus). BMC Genomics 2013, 14, 846.
- Clarke, T.H.; Garb, J.E.; Hayashi, C.Y.; Haney, R.A.; Lancaster, A.K.; Corbett, S.; Ayoub, N.A. Multi-tissue transcriptomics of the black widow spider reveals expansions, co-options, and functional processes of the silk gland gene toolkit. BMC Genomics 2014, 15, 365.
- Chaw, R.C.; Correa-Garhwal, S.M.; Clarke, T.H.; Ayoub, N.A.; Hayashi, C.Y. Proteomic evidence for components of spider silk synthesis from black widow silk glands and fibers. J. Proteome Res. 2015, 14, 4223–4231.
- Hu, X.; Kohler, K.; Falick, A.M.; Moore, A.M.; Jones, P.R.; Vierra, C. Spider egg case core fibers: Trimeric complexes assembled from TuSp1, ECP-1, and ECP-2. Biochemistry 2006, 45, 3506–3516.
- Larracas, C.; Hekman, R.; Dyrness, S.; Arata, A.; Williams, C.; Crawford, T.; Vierra, C.A. Comprehensive proteomic analysis of spider dragline silk from black widows: A recipe to build synthetic silk fibers. Int. J. Mol. Sci. 2016, 17, 1537.
- Vasanthavada, K.; Hu, X.; Falick, A.M.; La Mattina, C.; Moore, A.M.; Jones, P.R.; Yee, R.; Reza, R.; Tuton, T.; Vierra, C. Aciniform spidroin, a constituent of egg case sacs and wrapping silk fibers from the black widow spider Latrodectus hesperus. J. Biol. Chem. 2007, 282, 35088–35097.
- La Mattina, C.; Reza, R.; Hu, X.; Falick, A.M.; Vasanthavada, K.; McNary, S.; Yee, R.; Vierra, C.A. Spider minor ampullate silk proteins are constituents of prey wrapping silk in the cob weaver Latrodectus hesperus. Biochemistry 2008, 47, 4692–4700.
- He, Q.; Duan, Z.; Yu, Y.; Liu, Z.; Liang, S. The venom gland transcriptome of Latrodectus tredecimguttatus revealed by deep sequencing and cdna library analysis. PLoS ONE 2013, 8, e81357.
- Casem, M.L.; Collin, M.A.; Ayoub, N.A.; Hayashi, C.Y. Silk gene transcripts in the developing tubuliform glands of the western black widow, Latrodectus hesperus. J. Arachnol. 2010, 38, 99–103.
- Jeffery, F.; La Mattina, C.; Tuton-Blasingame, T.; Hsia, Y.; Gnesa, E.; Zhao, L.; Franz, A.; Vierra, C. Microdissection of black widow spider silk-producing glands. J. Vis. Exp. 2011, 47, e2382.
- Nentwig, W. Ecophysiology of Spiders; Springer: Berlin, Germany, 1987.
- Foelix, R. Biology of Spiders; Oxford University Press: New York, NY, USA, 1996.
- Guerette, P.A.; Ginzinger, D.G.; Weber, B.H.; Gosline, J.M. Silk properties determined by gland-specific expression of a spider fibroin gene family. Science 1996, 272, 112–115.
- Gatesy, J.; Hayashi, C.; Motriuk, D.; Woods, J.; Lewis, R. Extreme diversity, conservation, and convergence of spider silk fibroin sequences. Science 2001, 291, 2603–2605.
- Ayoub, N.A.; Garb, J.E.; Tinghitella, R.M.; Collin, M.A.; Hayashi, C.Y. Blueprint for a high-performance biomaterial: Full-length spider dragline silk genes. PLoS ONE 2007, 2, e514.
- Beckwitt, R.; Arcidiacono, S. Sequence conservation in the C-terminal region of spider silk proteins (spidroin) from Nephila clavipes (tetragnathidae) and Araneus bicentenarius (araneidae). J. Biol. Chem. 1994, 269, 6661–6663.
- Hayashi, C.Y.; Blackledge, T.A.; Lewis, R.V. Molecular and mechanical characterization of aciniform silk: Uniformity of iterated sequence modules in a novel member of the spider silk fibroin gene family. Mol. Biol. Evol. 2004, 21, 1950–1959.
- Trancik, J.E.; Czernuszka, J.T.; Bell, F.I.; Viney, C. Nanostructural features of a spider dragline silk as revealed by electron and X-ray diffraction studies. Polymer 2006, 47, 5633–5642.
- Jenkins, J.E.; Holland, G.P.; Yarger, J.L. High resolution magic angle spinning NMR investigation of silk protein structure within major ampullate glands of orb weaving spiders. Soft Matter 2012, 8, 1947–1954.
- Jenkins, J.E.; Creager, M.S.; Butler, E.B.; Lewis, R.V.; Yarger, J.L.; Holland, G.P. Solid-state NMR evidence for elastin-like beta-turn structure in spider dragline silk. Chem. Commun. 2010, 46, 6714–6716.
- Hieber, C.S. Spider cocoons and their suspension systems as barriers to generalist and specialist predators. Oecologia 1992, 91, 530–535.
- Tian, M.; Lewis, R.V. Molecular characterization and evolutionary study of spider tubuliform (eggcase) silk protein. Biochemistry 2005, 44, 8006–8012.
- Hu, X.; Kohler, K.; Falick, A.M.; Moore, A.M.; Jones, P.R.; Sparkman, O.D.; Vierra, C. Egg case protein-1. A new class of silk proteins with fibroin-like properties from the spider Latrodectus hesperus. J. Biol. Chem. 2005, 280, 21220–21230.
- Hu, X.; Lawrence, B.; Kohler, K.; Falick, A.M.; Moore, A.M.; McMullen, E.; Jones, P.R.; Vierra, C. Araneoid egg case silk: A fibroin with novel ensemble repeat units from the black widow spider, Latrodectus hesperus. Biochemistry 2005, 44, 10020–10027.
- Clarke, T.H.; Garb, J.E.; Haney, R.A.; Chaw, R.C.; Hayashi, C.Y.; Ayoub, N.A. Evolutionary shifts in gene expression decoupled from gene duplication across functionally distinct spider silk glands. Sci. Rep. 2017, 7, 8393.
More
