Nodal proteins show a different affinity to TGF-β receptors; it was demonstrated that the presence of binding proteins that act as partners enhances the signal cascade, improving the interactions between Nodal and its receptors. It was established that a fundamental obligatory co-receptor for the TGF-β family member Nodal is the cell surface glycosylphosphatidylinositol (GPI)-linked glycoprotein Cripto-1 (CR-1). Co-immunoprecipitation experiments show the interaction between Nodal and CR-1, supporting the idea that CR-1 acts as an obliged coreceptor for Nodal that potentiates its signal cascade.
- Nodal
- Cripto-1
- proliferation
- drug resistance
- biomarker
- therapeutic targets
1. Introduction
Nodal signal is transduced from the membrane to the nucleus through cooperation of proteins which activation is regulated by Nodal itself. Indeed, SMAD heterodimers bind to transcription factors that regulate Lefty genes transcription. Lefty proteins are antagonists of Nodal morphogens, switching off Nodal signal cascade and thus acting as negative feedback regulators [1].
Evidence shows that Nodal has signal activity even in the absence of CR-1 interacting directly with its receptor [2]. CR-1, also known as Teratocarcinoma-derived growth factor1 (TDGF-1), was firstly identified in teratocarcinoma cells NTERA2 [3]. It belongs to the EGF-CFC ( Cripto-1/FRL1/Cryptic) family that encodes a group of structurally related proteins that serve as important factors during early embryogenesis in vertebrates such as Xenopus , Zebrafish , mice, and humans [4]. Prior to gastrulation event, in the epiblast, CR-1 expression is symmetrical and uniform. Then, it forms an asymmetrical proximal-distal gradient, shifting caudally towards the region of the nascent primitive streak, regulating A-P patterning [5][6].
As other members of the EGF-CFC family, CR-1 contains an NH2-terminal signal peptide, a modified EGF-like region, a conserved cysteine-rich domain (CFC motif), and a short hydrophobic COOH-terminus that contains additional sequences for GPI cleavage and attachment that generate a biologically active soluble form [7]. Site-directed mutagenesis experiments showed that CR-1 directly interacts with ALK4 through its CFC domain and accommodates Nodal protein by interacting through its EGF-like motif. The binding between CR-1 and ALK4, and in particular the substrate-determining loop (L45) of ALK4, is fundamental for Nodal-induced SMAD2 activation. In fact, alterations of this loop are sufficient to redirect the Nodal signal from SMAD2 to SMAD1 activation through the ALK4/CR-1 complex [8]. In addition, Watanabe et al. showed that the GPI-signal sequence of CR-1 is necessary to induce optimum Nodal signaling in trans as well as in cis , demonstrating that both transmembrane and soluble CR-1 forms are active to induce Nodal signal cascade [9].
Recently, an additional role of these signal cascades was demonstrated in the maintaining of embryonic stem cell pluripotency [10], and in the sustaining of the cancer stem cell (CSC) compartments ( Figure 1 ), which is discussed in the next section.
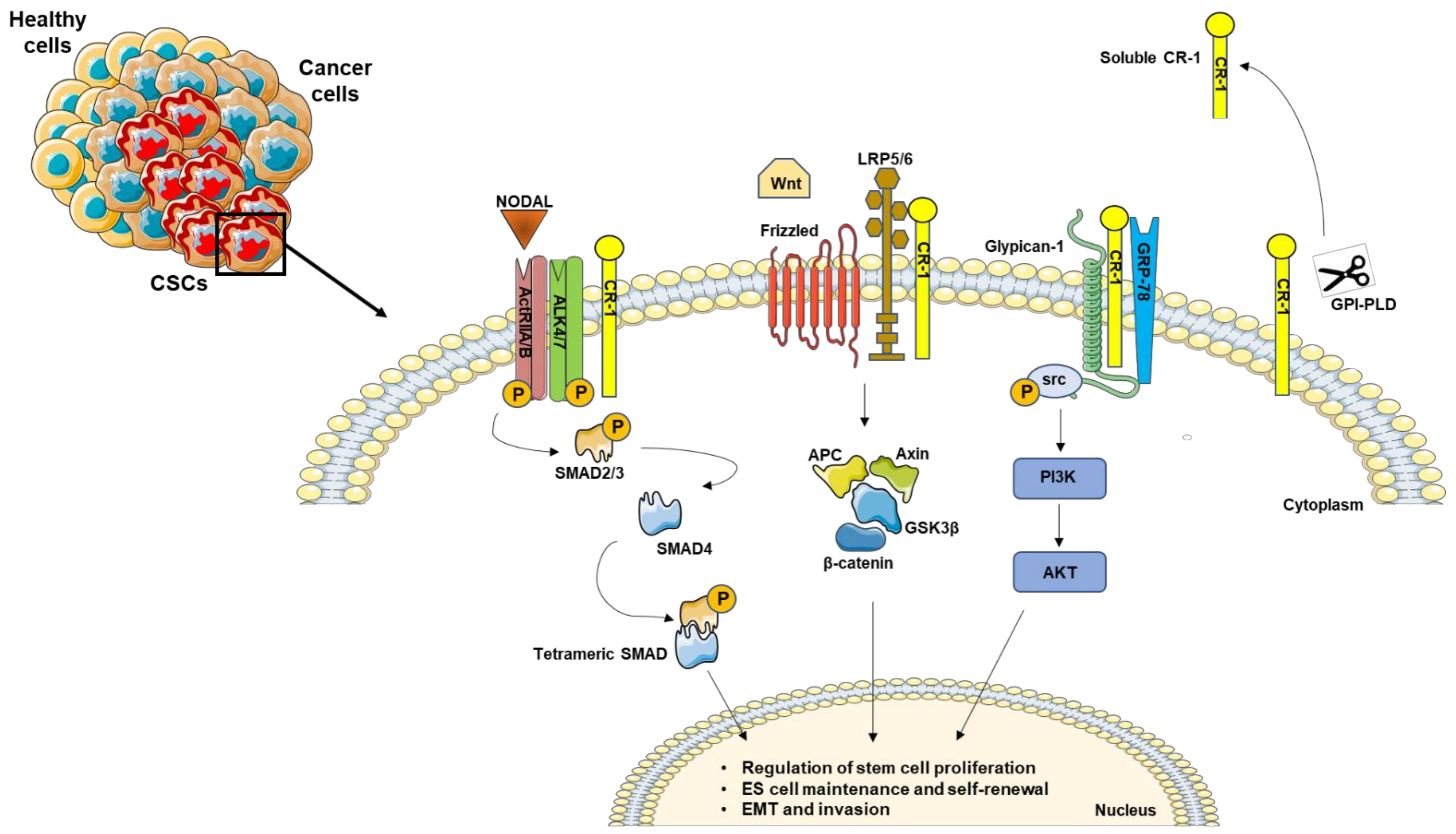 Figure 1. Schematic representation of Nodal/CR-1 signaling pathway in cancer stem cells. CR-1 acts as a cofactor for Nodal to activate downstream SMADs effectors. In the absence of Nodal, CR-1 can also signal in a non-canonical pathway through Frizzled and LRP4/5 complex, activating β-catenin signaling. In addition, CR-1 interacts with Glypican-1 and GRP78, triggering PIK3/AKT activation by Src phosphorylation. In addition, the GPI-specific phospholipase D (GPI-PLD) is able to cleave CR-1, generating a biological active soluble form.
Figure 1. Schematic representation of Nodal/CR-1 signaling pathway in cancer stem cells. CR-1 acts as a cofactor for Nodal to activate downstream SMADs effectors. In the absence of Nodal, CR-1 can also signal in a non-canonical pathway through Frizzled and LRP4/5 complex, activating β-catenin signaling. In addition, CR-1 interacts with Glypican-1 and GRP78, triggering PIK3/AKT activation by Src phosphorylation. In addition, the GPI-specific phospholipase D (GPI-PLD) is able to cleave CR-1, generating a biological active soluble form.
2. Nodal and Cripto-1 as Theranostic Targets
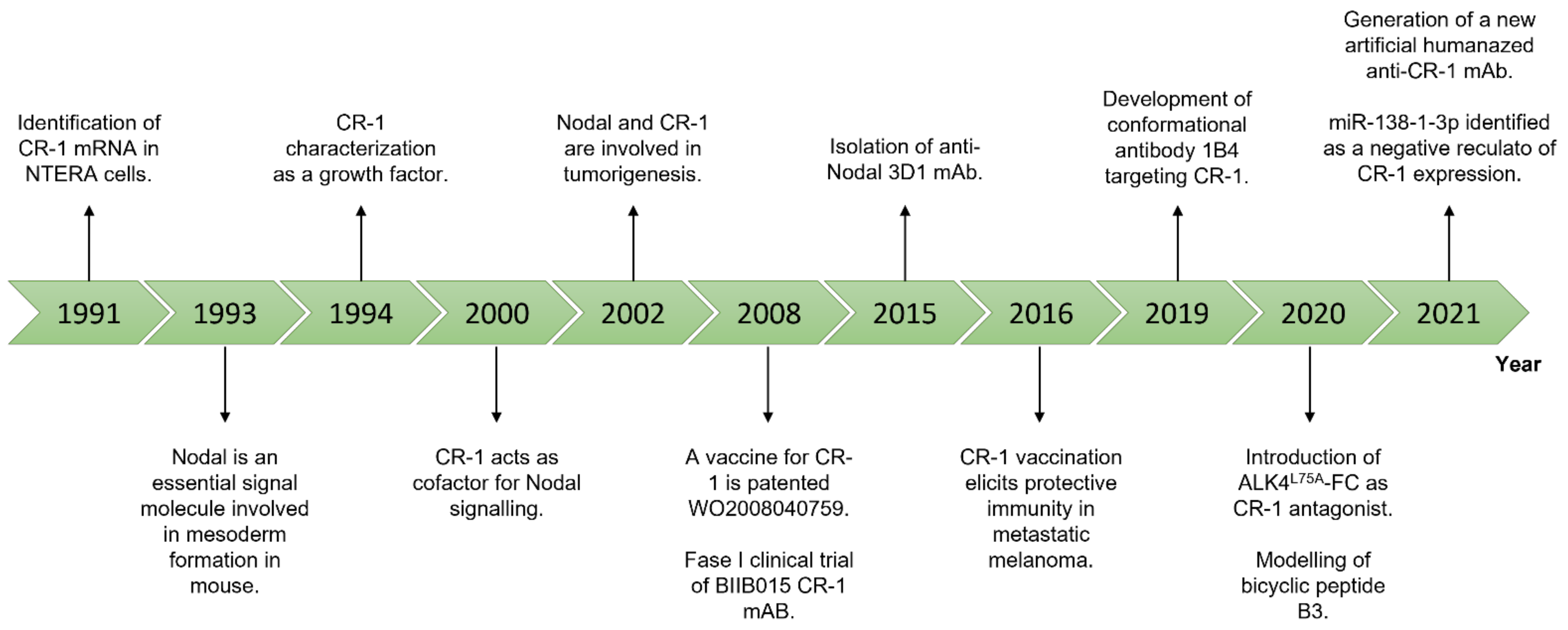
3. Conclusions
References
- Wei, S.; Wang, Q. Molecular regulation of Nodal signaling during mesendoderm formation. Acta Biochim. Biophys. Sin. (Shanghai). 2018, 50, 74–81.
- Liguori, G.L.; Borges, A.C.; D’Andrea, D.; Liguoro, A.; Gonçalves, L.; Salgueiro, A.M.; Persico, M.G.; Belo, J.A. Cripto-independent Nodal signaling promotes positioning of the A-P axis in the early mouse embryo. Dev. Biol. 2008, 315, 280–289.
- Ciccodicola, A.; Dono, R.; Obici, S.; Simeone, A.; Zollo, M.; Graziella Persico, M. Molecular characterization of a gene of the “EGF family” expressed in undifferentiated human NTERA2 teratocarcinoma cells. EMBO J. 1989, 8, 1987–1991.
- Salomon, D.S.; Bianco, C.; Ebert, A.D.; Khan, N.I.; De Santis, M.; Normanno, N.; Wechselberger, C.; Seno, M.; Williams, K.; Sanicola, M.; et al. The EGF-CFC family: Novel epidermal growth factor-related proteins in development and cancer. Endocr. Relat. Cancer 2000, 7, 199–226.
- Ding, J.; Yang, L.; Yan, Y.T.; Chen, A.; Desai, N.; Wynshaw-Boris, A.; Shen, M.M. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature 1998, 395, 702–707.
- Shen, M.M.; Schier, A.F. The EGF-CFC gene family in vertebrate development. Trends Genet. 2000, 16, 303–309.
- Ravisankar, V.; Singh, T.P.; Manoj, N. Molecular evolution of the EGF-CFC protein family. Gene 2011, 482, 43–50.
- Yeo, C.Y.; Whitman, M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol. Cell 2001, 7, 949–957.
- Watanabe, K.; Hamada, S.; Bianco, C.; Mancino, M.; Nagaoka, T.; Gonzales, M.; Bailly, V.; Strizzi, L.; Salomon, D.S. Requirement of glycosylphosphatidylinositol anchor of Cripto-1 for trans activity as a nodal co-receptor. J. Biol. Chem. 2007, 282, 35772–35786.
- Pauklin, S.; Vallier, L. Activin/nodal signalling in stem cells. Development 2015, 142, 607–619.
- Weinstein, I.B.; Joe, A. Oncogene addiction. Cancer Res. 2008, 68, 3077–3080.
- Barbato, L.; Bocchetti, M.; Di Biase, A.; Regad, T. Cancer Stem Cells and Targeting Strategies. Cells 2019, 8, 926.
- Al-Lazikani, B.; Banerji, U.; Workman, P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 2012, 30, 679–692.
- Sandomenico, A.; Ruvo, M. Targeting Nodal and Cripto-1: Perspectives Inside Dual Potential Theranostic Cancer Biomarkers. Curr. Med. Chem. 2018, 26, 1994–2050.
- Xu, C.H.; Wang, Y.; Qian, L.H.; Yu, L.K.; Zhang, X.W.; Wang, Q.B. Serum Cripto-1 is a novel biomarker for non-small cell lung cancer diagnosis and prognosis. Clin. Respir. J. 2017, 11, 765–771.
- Spiller, C.M.; Lobo, J.; Boellaard, W.P.A.; Gillis, A.J.M.; Bowles, J.; Looijenga, L.H.J. Cripto and MIR-371A-3P are serum biomarkers of testicular germ cell tumors and are detected in seminal plasma from azoospermic males. Cancers (Basel) 2020, 12, 760.
- Xue, Y.J.; Chen, S.N.; Chen, W.G.; Wu, G.Q.; Liao, Y.F.; Xu, J.B.; Tang, H.; Yang, S.H.; He, S.Y.; Luo, Y.F.; et al. Cripto-1 expression in patients with clear cell renal cell carcinoma is associated with poor disease outcome. J. Exp. Clin. Cancer Res. 2019, 38, 1–17.
- Liu, Q.; Cui, X.; Yu, X.; Bian, B.-S.-J.; Qian, F.; Hu, X.; Ji, C.; Yang, L.; Ren, Y.; Cui, W.; et al. Cripto-1 acts as a functional marker of cancer stem-like cells and predicts prognosis of the patients in esophageal squamous cell carcinoma. Mol. Cancer 2017, 16, 81.
- Bianco, C.; Strizzi, L.; Mancino, M.; Rehman, A.; Hamada, S.; Watanabe, K.; De Luca, A.; Jones, B.; Balogh, G.; Russo, J.; et al. Identification of Cripto-1 as a novel serologic marker for breast and colon cancer. Clin. Cancer Res. 2006, 12, 5158–5164.
- De Angelis, E.; Grassi, M.; Gullick, W.J.; Johnson, G.R.; Rossi, G.B.; Tempesta, A.; De Angelis, F.; De Luca, A.; Salomon, D.S.; Normanno, N. Expression of cripto and amphiregulin in colon mucosa from high risk colon cancer families. Int. J. Oncol. 1999, 14, 437–440.
