There are the following main assumptions of the Valence-shell Electron-pair Repulsion (VSEPR) model.
- The arrangement of covalent bonds of the atom centre analyzed depends on the number of electron pairs in its valence shell: bonds and nonbonding pairs as lone electron pairs.
- The arrangement of valence electron pairs around the centre considered is to maximize their distances apart.
- The non-valence electrons - inner electrons with nucleus (i.e. the core) possess the spherical symmetry (or at least it is in force for the main groups elements).
It is worth to note that the intra- and intermolecular interactions influence on electronic and molecular structures in accordance with this VSEPR model.
- valence-shell electron-pair repulsion model
- secondary bonds
- hypervalency
1. The VSEPR model, hypervalency and secondary bonds
In the case of the central atom that obeys the octet rule, the additional interaction (secondary bond) may lead to the increase of the electron charge at this centre and consequently to the hypervalency. One may refer to the 4e-3c structure (four electrons - three centres), marked as X-A∙∙∙B where the A-centre involved in a contact with B that donates the lone electron pair is hypervalent in the case of strong interactions.[3][5] The latter contact, i.e. the additional A∙∙∙B secondary bond is responsible for the hypervalency of the A-centre; in the isolated X-A species the A-centre obeyes the octet rule. The 4e-3c structure may be related to numerous interactions (secondary bonds) analysed in numerous studies, to hydrogen bonds, halogen and tetrel bonds, and many others.[5] The [FHF]- anion is an example of a very strong hydrogen bond [6][7] where the H-centre may be considered as the hypervalent one.[3] The hydrogen centre in this anion does not obey the duplet rule that is considered for H-centre (and He) instead of octet rule which is applied for other elements. It is worth noting that for systems of three atoms such as those discussed here the term of 3c/4e hypervalent ω-bond was introduced [8] (3c/4e designation follows ref.8). Thus the latter term of hypervalent bond is related to the hypervalency of the centre considered. This is why the secondary bonds, especially the strong ones, are equated with the hypervalent bonds where the interacting centre does not obey the octet rule. However one should remember that the term "hypervalent bond" (ω-bond) concerns three-centre system (X-A∙∙∙B) while the secondary bond two-centre system (A∙∙∙B) rather.
The hypervalency as an electronic structure phenomenon is discussed and questioned in some studies.[9][10] It was pointed out that for numerous centres that are usually indicated as the hypervalent ones, the number of electrons attributed to valence shell often only slightly exceeds, if it does at all, number eight. It was justified also that there are mechanisms that try to protect the octet or doublet structure of the central atom if this is the main group element or particularly H-atom, respectively.[5] These mechanisms lead most often to the outflow of the electron charge from the A-centre into other parts of the species acting as the Lewis acid unit (Scheme 1).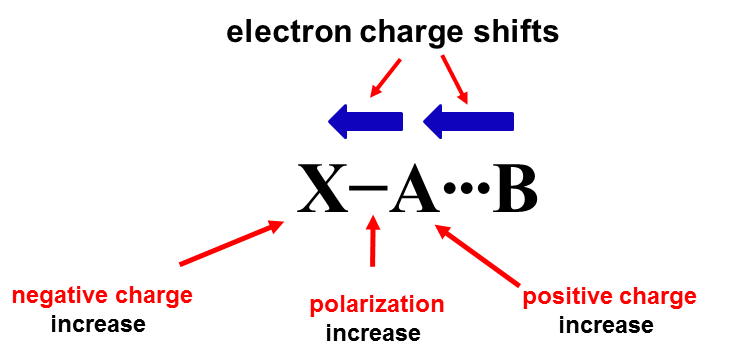
2. The classification of interactions
The hydrogen bond is an interaction that was analysed in various studies due to its crucial role in numerous physical, chemical and biological processes.[11] The halogen bond, another interaction, may be treated as the counterpart of the hydrogen bond.[12][13] This may be surprising since the electronegative halogen plays here a role of the Lewis acid centre.
One of explanations of dual character of halogens that may act as Lewis acid and Lewis base centres is based on the σ-hole concept.[14] According to this concept, the depletion of the electron charge in the elongation of bond to halogen results in the increase of electrostatic potential (EP) at the edge of halogen centre, up to positive values. This region is named as the σ-hole. Consequently, the excess of the electron charge in the perpendicular direction and near to this direction also occurs that is connected with the negative electrostatic potential. Scheme 2 illustrates the dual character of the halogen centres.
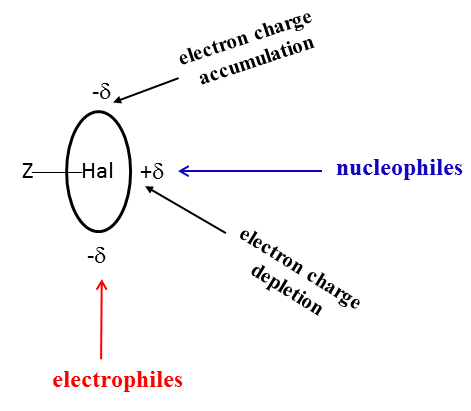
Scheme 2. The dual character of the halogen centre (Hal) which is connected by covalent bond with the Z-centre.
One can see that regions of the depletion and concentration of the electron charge occur for halogen centres; they correspond to their Lewis acid and Lewis base properties, respectively. The electron charge distribution presented in Scheme 2 has approximately an ellipsoidal shape. It is worth to note that the fluorine centres possess usually only characteristics of the Lewis bases, the cases they act as Lewis acid centres are rather rare.
A similar situation to this one described for halogens occurs for other elements of main groups. In the elongation of bonds to the elements of 16, 15, and 14 groups, the regions of the positive EP are observed that often results in interactions of these elements with the electron donors.[14] The corresponding interactions are named as the chalcogen, pnicogen, and tetrel bonds, respectively. For noble gas elements such positive EP regions are also observed which may interact with the Lewis base units through aerogen bonds.[15]
There are other regions of positive EP that may act as the Lewis acid sites. These are π-holes observed for some planar molecules or for planar fragments of molecules.[13][14] For example, such regions may occur at triel centers (13th group elements).[16] It is worth mentioning that the π-hole may be associated with a specific atom or with two or more atoms. For example, in benzene and its derivatives π-holes are craters above and below the rings.
The interactions related to groups of the periodic system were mentioned above here (tetrel bonds, chalcogen bonds, and others). However it is worth mentioning that the names related to single elements appear also from time to time in various studies. For example, the hydrogen bond, the lithium, carbon, fluorine, gold, beryllium, and magnesium bonds often occur. One can see that these names, both related to groups and to single elements, are connected with the Lewis acid properties of the centre involved in the interaction, which was discussed in one of the latest studies.[5] However, names related to the Lewis base properties of the centre being in a contact with the electron acceptor occur sometimes; one can mention hydride and halide bonds.
One can see that the classification of interactions is not systematic and uniform. One can have numerous reservations to classifications and names of interactions (secondary bonds). These reservations are shortly presented below here.
- The majority of names of interactions refers to the Lewis acid centres, less often some names concern the Lewis base centres.
- Some of names concern groups, and some of them only single elements.
- The properties of interactions within the same group of the periodic system may differ significantly. For example, halogen bond with the chlorine centre possesses different properties than such interaction with the iodine centre.
- There are numerous sub-classes within any interaction considered, for example, there are numerous sub-classes of the hydrogen bond related to the electron donor site that could be a single centre, multi-centre related to π-electrons or multicentre related to σ-electrons.
3. Secondary bonds and changes of conformations
The intra- or intermolecular interactions (secondary bonds) lead to structural changes that mainly concern conformations of centres being in contact. These structural modifications are greater for stronger interactions which are often classified as hypervalent bonds. Let us describe geometrical deformations related to changes of conformations which accompany formation of tetrel bonds. The tetrel bond is an interaction between the tetrel centre (14 group element) acting as the Lewis acid site and the electron donor. In the case of tetrahedral conformation corresponding to the sp3 hybridization of tetrel centre, there are four σ-holes in extensions of bonds to this centre. The interaction of tetrel species through such σ-hole with the electron donating ligand may lead, in the case of strong interaction, to the trigonal bipyramid conformation or to the structure possessing geometry close to this conformation.[5]
A similar situation occurs for the pnicogen (15 group element) cations characterized by the tetrahedral geometry which through σ-holes located at pnicogen centre may interact with electron donors; the latter may lead to the trigonal bipyramid geometry, similarly as it is observed for tetrel bonds. For both types of interactions, for neutral tetrel bonds and for charge assisted pnicogen bonds, numerous correlations were found which show that for stronger interactions the systems are closer to the trigonal bipyramid structure. For very weak interactions the tetrahedral structure is only slightly disturbed. Hence one can see that both interactions, the charge assisted pnicogen bond and the tetrel bond, which concern elements of different groups possess very similar characteristics.
The structural changes are also observed for the triel bonds. The triel centres are often located in planar molecules or in planar fragments of molecules and they are characterized by the regions of the positive electrostatic potentials, such regions are related usually to the π-holes. These triel centres may interact with electron donors that leads, in the case of strong interactions, to the change of the trigonal configuration of the triel centre into the tetrahedral structure.[16] These are only few examples of changes of configurations being a result of interactions.
4. Secondary bonds and the VSEPR model
Figure 1, as a whole, is of more general meaning since it presents the configurations predicted by the VSEPR approach where not only bonds that influence the molecular shapes are taken into account but also lone electron pairs. In other words valence electron pairs are taken into account. The AXnEm designations applied in this figure should be understood in the same way, as it was described earlier here. To simplify these designations, the differences between n ligands (marked as X) linked with A-centre are not specified.
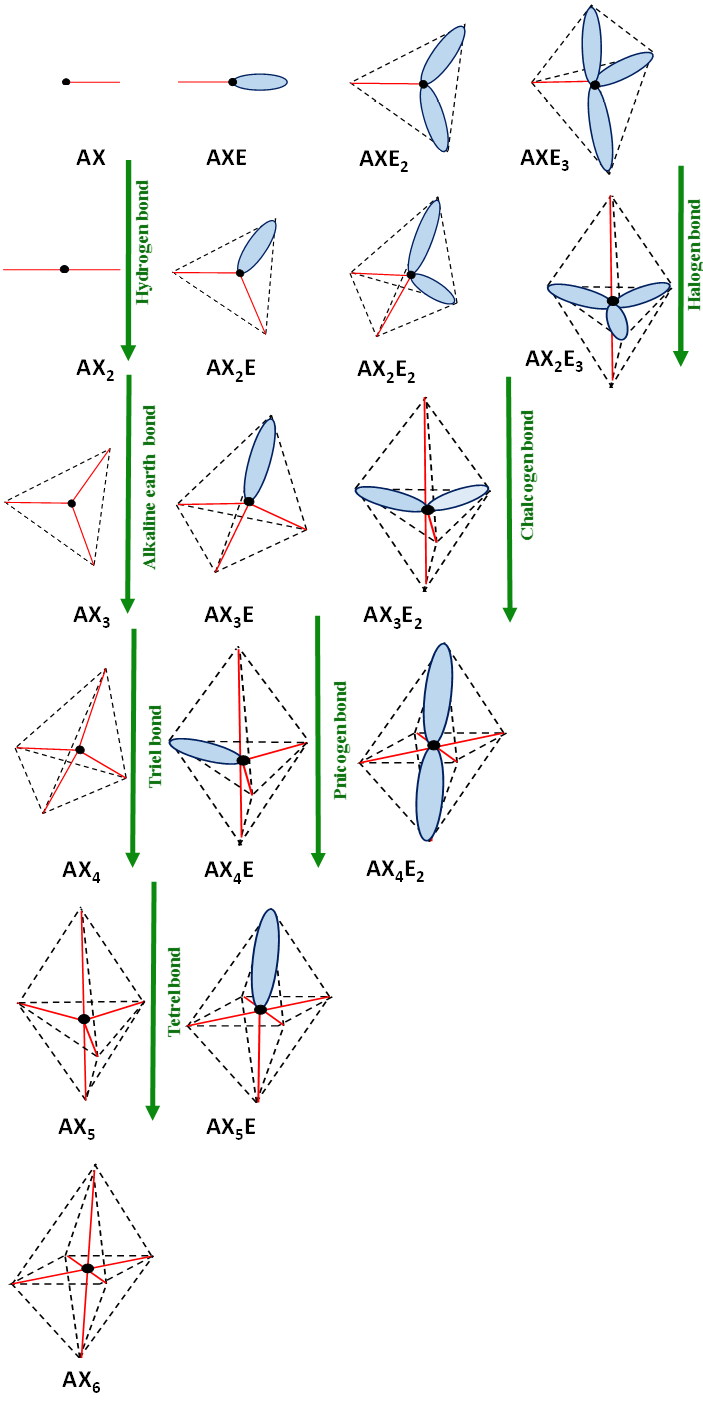
Figure 1. The VSEPR structures and their interrelations with interactions. The lone electron pairs presented as ellipsoids do not correspond to their shapes, they are introduced only to show schematically their localizations according to VSEPR model. The bonds are designated by red solid lines. The black circle corresponds to the centre considered, AXnEm marks the type of structure where n and m subscripts correspond to the number of ligands (X) and electron pairs (E), respectively (n or m is omitted if it is equal to one). The green arrows show interactions that change one of structures into another one.
Few interactions are involved in Figure 1. The hydrogen and halogen bonds are included there. Let us look at other interactions. For example, the change of the tetrahedral (Td) AX2E2 configuration into the AX3E2 (TBP) may be realized through the chalcogen bond. There are numerous examples of such a situation. As the interaction of SFCl possessing the AX2E2 configuration with Cl- anion that leads to the SFCl2- anion. The latter is a very strong interaction thus the product of this reaction (interaction) is characterized by the AX3E2 configuration of the trigonal bipyramid where lone electron pairs are located in the plane of the triangle. However, it is more often observed that the chalcogen bond leads to the change of AX2E2 into the geometry being intermediate between Td (AX2E2) and TBP (AX3E2). The same concerns other interactions; the change of AX4 into AX5 (Td into TBP) may be realized only by the extremely strong tetrel bond where the trigonal bipyramid structure corresponds to the transition state of the SN2 reaction.[5] The ClCH3∙∙∙∙F- tetrel interaction is an example that is the preliminary stage of the SN2 reaction. The AX4 configuration of the ClCH3 species changes into the AX5 configuration of ClCH3F- transition state and next for products, Cl-∙∙∙∙CH3F, the CH3F possesses the AX4 configuration.
It is worth mentioning that not only the tetrel bond may realize the AX4 → AX5 change. It may be also the charge assisted pnicogen bond described in the former section. The same concerns other interactions indicated in Figure 1. Each of changes shown in this figure is realized by various types of interactions in various types of structures. The following changes of structures were shown for the interactions usually characterized as the σ-hole bonds; halogen, chalcogen, pnicogen, and tetrel bonds; AXE3 → AX2E3, AX2E2 → AX3E2, AX3E → AX4E and AX4 → AX5, respectively. The AX3 → AX4 change may be realized by the triel bond while the AX2 → AX3 by the beryllium bond or other alkaline earth bonds. However, as it was noted above here, each of these changes of conformations may be realized by at least few different interactions (secondary bonds). Similarly, each of interactions may realize various changes of conformations. For example, the halogen bond if concerns the multivalent halogen centre may concern different changes of structures from those mentioned earlier here (i.e., from AXE3 → AX2E3 change typical for the monovalent halogens).[17]
It is also worth to note that conformations presented in Figure 1 often do not correspond to real electronic structures. However, their changes described earlier here and particularly their interactions are well fitted into the structures predicted by the VSEPR approach (Figure.1). The structure of water is an example since the sp3 hybridization model is often presented with the oxygen located in the centre of the tetrahedron and both two OH bonds and two lone electron pairs directed towards the vertices. Numerous structural properties of water clusters as well as numerous crystal structures containing water molecules are in line with this model. It is worth noting, however, that this is not true picture of the water molecule; two lone electron pairs are not equivalent and the sp3 hybridization model is not correct.[18]
References
- Gillespie, R.J.; Hargittai, I.. The VSEPR model of molecular geometry.; Dover Publications: New York, 2012; pp. 40-44.
- Martin, J.C.; “Frozen” Transition States: Pentavalent Carbon et al.. Science 1983, 221, 509-514, DOI: 10.1126/science.221.4610.509.
- Crabtree, R.H.; Hypervalency, secondary bonding and hydrogen bonding: Siblings under the skin. . Chem. Soc. Rev. 2017, 46, 1720-1729, DOI: 10.1039/C6CS00688D .
- Alcock, N.W; Secondary bonding to nonmetallic elements. Adv. Inorg. Chem. Radiochem. 1972, 15, 1-58, https://doi.org/10.1016/S0065-2792(08)60016-3.
- Grabowski, S.J.; Hydrogen bonds, and σ-hole and π-hole bonds – mechanisms protecting doublet and octet electron structures. Phys.Chem.Chem.Phys. 2017, 19, 29742–29759, DOI: 10.1039/C7CP06393H.
- Pylaeva, S.A.; Elgabarty, H.; Sebastiani, D.; Tolstoy, P.M.; Symmetry and dynamics of FHF- anion in vacuum, in CD2Cl2 and in CCl4. Ab initio MD study of fluctuating solvent–solute hydrogen and halogen bonds . Phys. Chem. Chem. Phys. 2017, 19, 26107-26120, DOI: 10.1039/c7cp04493c .
- Grabowski, S.J.; [FHF]--The Strongest Hydrogen Bond under the Influence of External Interactions. Crystals 2016, 6, 3, https://doi.org/10.3390/cryst6010003.
- Weinhold, F.; Landis, C.. Valency and Bonding, a Natural Bond Orbital Donor—Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005; pp. 275-306.
- Gillespie, R.J.; Silvi, B.; The octet rule and hypervalence: Two misunderstood concepts. Coord. Chem. Rev. 2002, 233-234, 53-62, https://doi.org/10.1016/S0010-8545(02)00102-9.
- Noury, S.; Silvi, B.; Gillespie, R.J.; Chemical Bonding in Hypervalent Molecules: Is the Octet Rule Relevant?. Inorg. Chem. 2002, 41, 2164–2172, https://doi.org/10.1021/ic011003v.
- Jeffrey, G.A.; Saenger, W.. Hydrogen Bonding in Biological Structures; Springer: Berlin, 1991; pp. 394-422.
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G.; The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601, https://doi.org/10.1021/acs.chemrev.5b00484.
- Politzer, P.; Murray, J.S.; Clark, T.; Halogen bonding and other σ-hole interactions: A perspective. Phys.Chem. Chem. Phys. 2013, 15, 11178–11189, DOI: 10.1039/C3CP00054K .
- Politzer, P.; Murray, J.S.; Clark, T.; Halogen bonding: An electrostatically-driven highly directional noncovalent interaction.. Phys. Chem. Chem. Phys. 2010, 12, 7748–7758, DOI: 10.1039/C004189K.
- Bauzá, A.; Frontera, A.; Aerogen Bonding Interaction: A New Supramolecular Force?. Angew. Chem. Int. Ed. 2015, 54, 7340-7343, https://doi.org/10.1002/anie.201502571.
- Grabowski, S.J.; Triel bond and coordination of triel centres–Comparison with hydrogen bond interaction.. Coord. Chem. Rev. 2020, 407, 213171, https:// doi.org/10.1016/j.ccr.2019.213171.
- Grabowski, S.J.; Halogen bond with the multivalent halogen acting as the Lewis acid center. Chem. Phys. Lett. 2014, 605-606, 131-136, https://doi.org/10.1016/j.cplett.2014.05.029.
- Clauss, A.D.; Nelsen, S.F.; Ayoub, M.; Moore, J.W.; Landis, C.R.; Weinhold, F.; Rabbit-ears hybrids, VSEPR sterics, and other orbital anachronisms.. Chem. Educ. Res. Pract. 2014, 15, 417-434, DOI https://doi.org/10.1039/C4RP00057A .
