Porcine reproductive and respiratory syndrome (PRRS) affects farmed pigs worldwide and still causes heavy direct and indirect losses. The syndrome emerged in the late 1980s, in USA, and later on in Europe, and it eventually became enzootic in most countries among farmed pigs. Late-term reproductive failure in sows with transplacental transmission of the virus, preweaning mortality of piglets, respiratory distress, anorexia, and possible cutaneous hyperemia in weaners and growers are common clinical signs of PRRS.
- pig
- PRRS
- PRRS virus
- immune response
- disease resistance
- disease control
1. Overview
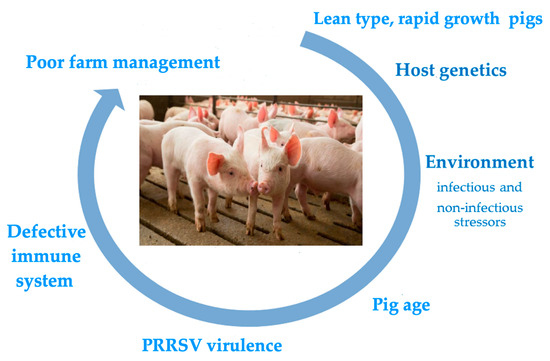
The control of porcine reproductive and respiratory syndrome (PRRS) is still a major issue worldwide in the pig farming sector. Despite extensive research efforts and the practical experience gained so far, the syndrome still severely affects farmed pigs worldwide and challenges established beliefs in veterinary virology and immunology. The clinical and economic repercussions of PRRS are based on concomitant, additive features of the virus pathogenicity, host susceptibility, and the influence of environmental, microbial, and non-microbial stressors. This makes a case for integrated, multi-disciplinary research efforts, in which the three types of contributing factors are critically evaluated toward the development of successful disease control strategies. These efforts could be significantly eased by the definition of reliable markers of disease risk and virus pathogenicity. As for the host’s susceptibility to PRRSV infection and disease onset, the roles of both the innate and adaptive immune responses are still ill-defined. In particular, the overt discrepancy between passive and active immunity and the uncertain role of adaptive immunity vis-à-vis established PRRSV infection should prompt the scientific community to develop novel research schemes, in which apparently divergent and contradictory findings could be reconciled and eventually brought into a satisfactory conceptual framework.
2. Porcine Reproductive and Respiratory Syndrome
3. Biosecurity: the Foundation of Successful Disease Control Strategies
4. Acclimatization as the Second Pillar of Successful Disease Control on the Farm
5. Which Elements Underlie Successful Disease Control?
6. Conclusions
References
- Holtkamp, D.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J. Swine Health Prod. 2013, 21, 72–84.
- Zimmerman, J.; Benfield, D.A.; Murtaugh, M.P.; Osorio, F.; Stevenson, G.W.; Tottemorell, M. Porcine reproductive and Respiratory Syndrome Virus (Porcine Arterivirus). Dis. Swine 2006, 9, 387–417.
- Crisci, E.; Fraile, L.; Montoya, M. Cellular Innate Immunity against PRRSV and Swine Influenza Viruses. Vet. Sci. 2019, 6, 26.
- Amadori, M.; Razzuoli, E. Immune Control of PRRS: Lessons to be Learned and Possible Ways Forward. Front. Vet. Sci. 2014, 1, 2.
- Bøtner, A. Diagnosis of PRRS. Vet. Microbiol. 1997, 55, 295–301.
- Turlewicz-Podbielska, H.; Włodarek, J.; Pomorska-Mól, M. Noninvasive strategies for surveillance of swine viral diseases: A review. J. Vet. Diagn Investig. 2020, 32, 503–512.
- Corzo, C.A.; Mondaca, E.; Wayne, S.; Torremorell, M.; Dee, S.; Davies, P.; Morrison, R.B. Control and elimination of porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154, 185–192.
- Holtkamp, D.J.; Polson, D.D.; Torremorell, M.; Morrison, B.; Classen, D.M.; Becton, L.; Henry, S.; Rodibaugh, M.T.; Rowland, R.R.; Snelson, H.; et al. Terminology for classifying swine herds by porcine reproductive and respiratory syndrome virus status. Tierarztl Prax. Ausg. G. Grosstiere Nutztiere 2011, 39, 101–112.
- Klinge, K.L.; Vaughn, E.M.; Roof, M.B.; Bautista, E.M.; Murtaugh, M.P. Age-dependent resistance to Porcine reproductive and respiratory syndrome virus replication in swine. Virol. J. 2009, 6, 177.
- Vashisht, K.; Erlandson, K.R.; Firkins, L.D.; Zuckermann, F.A.; Goldberg, T.L. Evaluation of contact exposure as a method for acclimatizing growing pigs to porcine reproductive and respiratory syndrome virus. J. Am. Vet. Med. Assoc. 2008, 232, 1530–1535.
- Singleton, H.; Graham, S.P.; Bodman-Smith, K.B.; Frossard, J.P.; Steinbach, F. Establishing Porcine Monocyte-Derived Macrophage and Dendritic Cell Systems for Studying the Interaction with PRRSV-1. Front. Microbiol. 2016, 7, 832.
- Bordon, Y. Macrophages: Innate memory training. Nat. Rev. Immunol. 2014, 14, 713.
- Lewis, C.R.; Ait-Ali, T.; Clapperton, M.; Archibald, A.L.; Bishop, S. Genetic perspectives on host responses to porcine reproductive and respiratory syndrome (PRRS). Viral Immunol. 2007, 20, 343–358.
- Brambilla, G.; Civitareale, C.; Ballerini, A.; Fiori, M.; Amadori, M.; Archetti, L.I.; Regini, M.; Betti, M. Response to oxidative stress as a welfare parameter in swine. Redox Rep. 2002, 7, 159–163.
- van Gucht, S.; van Reeth, K.; Pensaert, M. Interaction between porcine reproductive-respiratory syndrome virus and bacterial endotoxin in the lungs of pigs: Potentiation of cytokine production and respiratory disease. J. Clin. Microbiol. 2003, 41, 960–966.
- Zhiping, W.; Malmberg, P.; Larsson, B.M.; Larsson, K.; Larsson, L.; Saraf, A. Exposure to bacteria in swine-house dust and acute inflammatory reactions in humans. Am. J. Respir. Crit. Care Med. 1996, 154, 1261–1266.
- Boddicker, N.; Waide, E.H.; Rowland, R.R.; Lunney, J.K.; Garrick, D.J.; Reecy, J.M.; Dekkers, J.C. Evidence for a major QTL associated with host response to porcine reproductive and respiratory syndrome virus challenge. J. Anim. Sci. 2012, 90, 1733–1746.
- Burkard, C.; Opriessnig, T.; Mileham, A.J.; Stadejek, T.; Ait-Ali, T.; Lillico, S.G.; Whitelaw, C.B.A.; Archibald, A.L. Pigs Lacking the Scavenger Receptor Cysteine-Rich Domain 5 of CD163 Are Resistant to Porcine Reproductive and Respiratory Syndrome Virus 1 Infection. J. Virol. 2018.
- Broom, D.M. Animal welfare: Concepts and measurement. J. Anim. Sci. 1991, 69, 4167–4175.
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154.
