Lung cancer is one of the most frequent and most deadly types of solid cancers worldwide, and a highly complex, very heterogeneous disease. Histological classification divides lung cancers into non-small cell lung carcinoma (NSCLC, approximately 85% of the cases) and small cell lung carcinoma (SCLC, approximately 15% of the cases). NSCLC can be classified further into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Although lung cancer is treatable with early-stage radical interventions, it remains challenging because >70% of patients relapse and expire
[1][2][3][4][1,2,3,4]. In addition, the broad use of cytotoxic chemotherapies in lung cancer has reached a plateau
[5]. In the past decade, exploring cancer treatment options has led to alternative therapies, such as immunotherapies and targeted therapies
[6]. Cancer immunotherapies mainly target particular immune system components, such as immune-suppressive networks in the tumor microenvironment (TME), to enhance antitumor activity. Cancer cells shape their microenvironment and generate immune-suppressive networks, which finally overwhelm immunity and tumor progression
[7]. Tumor cells and stromal cells, such as tumor-associated macrophages (TAMs), create an immunosuppressive environment by secreting immune-suppressive factors, such as programmed cell death ligand (PDL) 1
[8]. Additionally, the TME disturbs production of tumor-specific T cells like cytotoxic T cells (CTLs), and generates immunosuppressive leukocytes, regulatory T cells (Tregs), and myeloid-derived suppressor cells
[9][10][9,10]. Various immune checkpoint inhibitors, such as CTL-associated protein 4 (CTL-4) and programmed cell death 1 (PD1), have been developed in immune-based therapies in different types of cancer
[11][12][11,12]. Although immune checkpoint inhibitors provide durable responses to various cancers, they are effective in only a subset of patients
[13]. Furthermore, recent advances in cancer research have led to molecular targeted therapies targeting identified gene mutations and molecular alterations to treat cancer
[14]. Genomic profiling with advanced techniques like next-generation sequencing has identified molecular alterations and driver mutations in lung cancer
[15]. The majority of genetic aberrations are in the epidermal growth factor receptor (EGFR), Kirsten rat sarcoma viral (KRAS), tyrosine-protein kinase MET (MET), anaplastic lymphoma (ALK), PI3KCA, ERBB2, and BRAF. EGFR and KRAS mutations are the most frequently found mutations in lung cancer. These specific mutations support tumor growth and proliferation by activating several signaling pathways
[16]. These findings recently led to the development of targeted agents like T tyrosine kinase inhibitors (TKI) to target EGFR-activating mutation and to use targeted-based therapies in lung cancer. However, only about 20% of patients benefit from these targeted therapies in lung cancer patients with drug-sensitive mutations. Furthermore, drug resistance caused by genetic alterations is a major impediment to long-term therapeutic outcomes
[17][18][17,18]. As a result, more in-depth research is required to develop new cancer-targeted medicines.
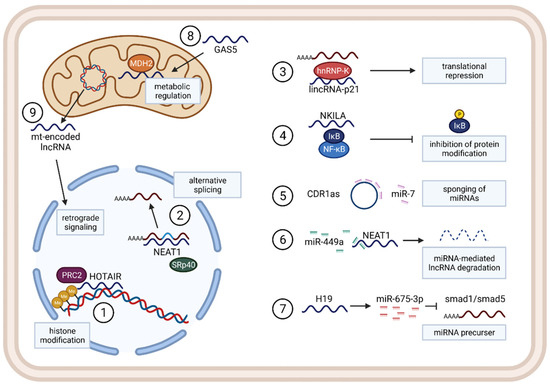
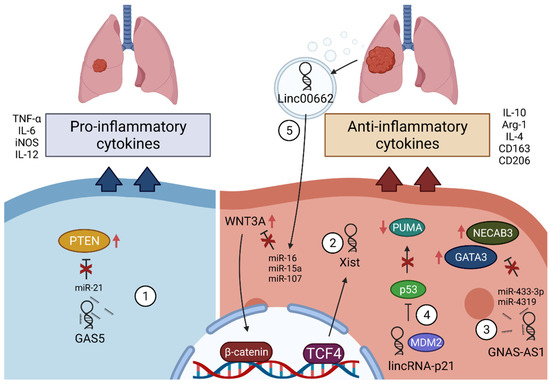
Non-coding RNAs, such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), are commonly dysregulated in cancer through the transcriptional, post-transcriptional, and epigenetic changes and have been shown to have significant roles in cancer initiation and progression
[19][20][19,20]. Deep sequencing and microarray profiling investigations have further shown that deregulation of lncRNA expression is also a critical factor in initiation and progression of lung cancer
[21][22][21,22]. Some recent studies also found that lncRNAs can influence TAM function in various ways
[23][24][25][23,24,25]. However, understanding lncRNA dysregulation and function in cancer is still in its early stages. In this present review, we will first briefly discuss activation and polarization of TAMs and their role in lung cancer progression, and then specially focus on lncRNAs’ functions and mechanisms, influence of lncRNAs on TAMs, their roles in TME inflammation, and regulation pathways in lung cancer. Finally, we will discuss the therapeutic potential of lncRNAs in diseases, particularly lung cancer.