1. Introduction
Lymphatic vessels serve as a drainage system for collecting and returning interstitial tissue fluid and protein (lymph) to the bloodstream
[1]. In addition, lymphatics provide an important pathway for leukocytes during an immune response
[2]. On the flip side, immune cells can regulate lymphatic vessel development, growth and function. Recruitment and activation of immune cells in the context of inflammation promotes lymphangiogenesis and also increases lymph flow
[3]. These effects amplify cell trafficking to lymph nodes and are important for mounting an effective immune response.
Under homeostatic conditions, neutrophils patrol the lymph nodes that drain the skin, lungs and gastrointestinal tract
[4]. They enter the lymph nodes via high endothelial venules (HEVs) in an L-selectin-dependent manner and reside within the lymph node interstitium
[5]. Neutrophil lymph node egress via efferent lymphatics is mediated by sphingosine-1-phosphate receptor (S1PR)
[5].
At the onset of inflammation (e.g., infection, tissue damage, and cancer), neutrophils rapidly migrate to sites of injury, where they can destroy microorganisms through phagocytosis, release anti-microbial molecules and promote immune cell recruitment
[6]. Once neutrophils enter inflamed tissues, they have several possible fates: most neutrophils are thought to die at the site of inflammation; however, a proportion can reverse transmigrate back into blood vessels
[7][8][7,8]. Furthermore, some neutrophils can egress sites of inflammation by entering lymphatic vessels and migrating to draining lymph nodes. This lymphatic migration of neutrophils has been observed in response to bacterial and parasitic infections and after vaccination
[9][10][11][12][9,10,11,12].
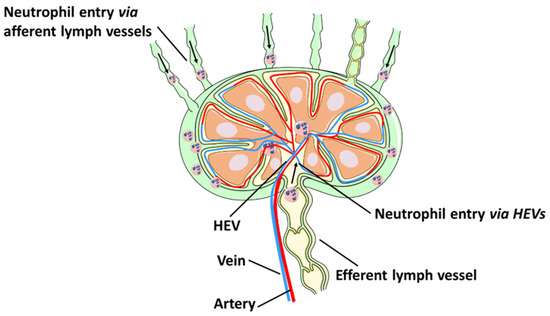
In addition to the lymphatic route, neutrophils can also enter draining lymph nodes from circulation via HEVs (
Figure 1). Neutrophil entry into the lymph node through HEVs has been observed following intradermal injection of
S. aureus [13] and Complete Freund’s Adjuvant (CFA)
[11] as well as in a model of tumour cell lysis in murine skin
[14]. A recent study showed that the majority of neutrophils were recruited to the lymph node directly from HEVs
[15], while a smaller population of neutrophils migrated directly from the site of inflammation to lymph nodes via afferent lymphatics
[15].
Figure 1. Neutrophil migration to lymph nodes. Neutrophils rapidly migrate from the site of inflammation into lymphatic vessels and draining lymph nodes. Neutrophils can also enter the lymph nodes from circulation by crossing HEVs.
Lymph node neutrophils are phenotypically distinct from circulating neutrophils and have the capacity to modulate adaptive immunity
[4]. Although the existence of bona fide neutrophil subsets remains to be conclusively demonstrated, the two different routes of neutrophil entry (via afferent lymphatics or HEVs) suggest that the two populations of lymph node neutrophils may have distinct functions. Neutrophils entering the lymph node via afferent lymphatics are arriving from sites of inflammation, are highly activated, express costimulatory molecules and carry tissue antigens
[9][10][9,10]. This may enable them to act as antigen presenting cells and to shuttle pathogens to draining lymph nodes. On the other hand, neutrophils arriving from circulation are likely coming from the bone marrow (although it is possible that a proportion of tissue neutrophils undergoes reverse transmigration to enter circulation
[7][8][7,8] and then migrates to draining lymph nodes) in response to pathogens in the lymph nodes. It is likely that neutrophils entering from circulation play a prominent role in preventing the systemic dissemination of bacteria
[15].
Another role of neutrophils in interacting with the lymphatic system is to contribute to lymphangiogenesis, a process which can modulate inflammation by controlling cell trafficking and antigen transport
[3][16][3,16]. Neutrophils mediate this function by releasing lymphangiogenic molecules, such as VEGF-D, at the site of inflammation
[17][18][17,18]. Here, we review neutrophil trafficking within the lymphatic system and the effect on the immune response, highlighting new evidence of neutrophils’ role in lymphangiogenesis.
2. Neutrophil Contributions to Lymphangiogenesis
Emerging evidence suggests that neutrophils may also interact with the lymphatic system by regulating lymphangiogenesis (or growth of new lymphatic vessels) in inflamed tissues and lymph nodes during inflammation and cancer progression
[3][19][3,95]. Recent studies have identified signals which promote the formation of the lymphatic vasculature
[20][21][96,97]. Vascular endothelial growth factors, such as VEGF-A, VEGF-C and VEGF-D can induce LEC sprouting and proliferation. VEGFs bind with different specificity to three endothelial transmembrane receptors: VEGFR-1, VEGFR-2, and VEGFR-3. The best characterized axis in lymphatic expansion and development involves VEGFR-3 and its ligands, VEGF-C and VEGF-D
[22][98].
The functional consequences of inflammation-induced lymphangiogenesis require further investigation. In some settings, expansion of the lymphatic network may support inflammation by promoting the transport of immune cells and antigens to the lymph node and enhancing the immune response. In other settings, lymphatic vessel expansion contributes to resolution of inflammation by draining inflammatory mediators and cells from the site of inflammation
[23][99]. For instance, inhibiting the lymphatic vasculature led to increased inflammation in mouse models of skin inflammation
[24][25][100,101], inflammatory bowel disease
[26][27][102,103] and rheumatoid arthritis
[28][104]. However, inhibiting this process improves graft survival in corneal transplantation
[29][30][105,106].
Unlike the well-established role for neutrophils in promoting blood vessel formation (angiogenesis) by producing VEGFs
[31][107], neutrophil contribution to lymphangiogenesis is only just beginning to be uncovered
[32][22]. Neutrophils secrete VEGF-D at sites of inflammation, which promotes proliferation of LECs, stimulates lymphangiogenesis
[33][108] and plays an important role during the development of the lymphatic system
[34][109]. Neutrophils may also increase VEGF-A bioavailability
[18]. In fact, after secretion VEGF becomes bound to the ECM and the interaction between VEGF-A and matrix proteins is mediated through the carboxy terminal region, also known as the heparin-binding or ECM-binding domain
[35][110]. This domain is able to bind heparin sulphate proteoglycans and other matrix proteins
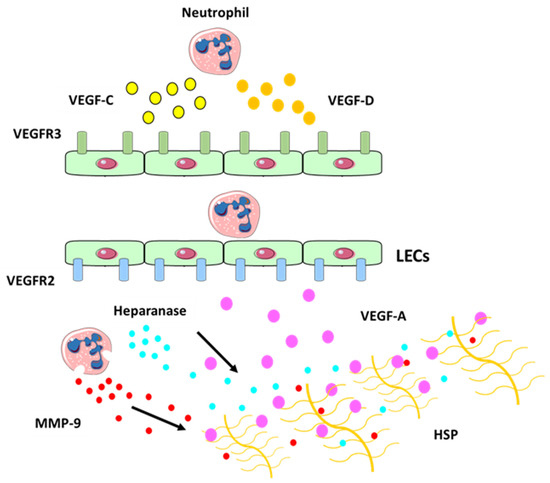
[35][36][110,111] and is thought to be responsible for the sequestration of VEGF-A within the matrix. Neutrophils act via MMP-9 and heparanase (which cleaves heparan sulphate preoteoglycans (HSP) side chains and releases the HSP-trapped VEGF-A) (
Figure 4) to increase the amount of biologically active VEGF-A, which in turn activates inflammatory lymphangiogenesis
[18].
Figure 4. Neutrophil contribution to inflammatory lymphangiogenesis. Schematic illustrating neutrophil contributions to lymphangiogenesis at the site of inflammation. Neutrophils can secrete VEGF-C (in yellow) and VEGF-D (in orange) at the site of inflammation which bind to their receptor VEGFR-3 (in green) expressed on LECs. Neutrophils can also increase VEGF-A (in purple) availability by secreting MMP-9 and heparanase which cleaves heparan sulphate preoteoglycans (HSP) side chains and release the HSP-trapped VEGF-A. This binds to VEGFR-2 receptor (in blue), which promotes the expansion of the lymphatic vessel network
[37][119].
Neutrophils may also promote tissue lymphangiogenesis by cooperating with macrophages
[18][38][18,112], as both macrophages and neutrophils contributed to inflammatory lymphangiogenesis in zebrafish by expressing VEGF-A, VEGF-C and VEGF-D
[39][113]. Neutrophils also expressed VEGF-D in the mucosa and lumen of inflamed airways
[17]. Furthermore, a study of lymphangiogenesis in abdominal aortic aneurysm (AAA) reported neutrophil infiltration around lymphatic microvessels
[40][114].
Similarly to tissue lymphangiogenesis, VEGF-A-VEGFR2 and VEGF-C/D-VEGFR3 are the principal mediators of lymph node (or intranodal) lymphangiogenesis
[41][115]. During inflammation, B cells
[42][116], macrophages recruited from peripheral tissues
[43][117] and fibroblast-type reticular stromal cells
[44][118] are the major sources of VEGF-A in the lymph node. Emerging evidence suggests that neutrophils also contribute to lymph node lymphangiogenesis
[18]. In mice lacking B cells, neutrophils compensate to support lymph lymphangiogenesis during prolonged inflammation
[18]. However, more studies are needed to determine whether specific neutrophil subsets are involved in this process.
Together, these studies support the emerging concept of neutrophils as important contributors to lymphangiogenesis in inflamed tissues and draining lymph nodes via the secretion of lymphangiogenic factors and LEC stimulation during the early and later stages of inflammation.
3. Conclusions and Perspectives
The last four decades have witnessed remarkable progress in
theour understanding of how immune cells traverse vast distances between tissues and secondary lymphoid organs to engage the innate and adaptive immune systems and mount an effective immune response. More recently, neutrophil lymphatic migration has been shown to be an important contributor to this process. This led to a paradigm shift in
the entryour view of neutrophils: from short-lived effectors designed to provide sterilizing immunity at sites of infection, to multifunctional immune cells that activate adaptive immunity in draining lymph nodes
[45][46][120,121]. The rapid entry of neutrophils into lymphatic vessels and their subsequent transmigration to lymph nodes places these leukocytes in prime position to provide early signals to adaptive immune cells
[9][10][11][12][9,10,11,12].
Neutrophils entering lymph nodes via the two different routes (HEVs versus afferent lymphatics) appear to have distinct phenotypes and functions. The majority of neutrophils arriving in draining lymph nodes are recruited from the circulation in response to inflammation in the lymph node and play an important role in preventing further pathogen dissemination
[15][47][15,122]. Neutrophils entering through the afferent lymphatics make up a smaller proportion of lymph node neutrophils but have an activated APC-like phenotype and often transport antigens from infected tissues
[9][10][9,10], suggesting that they may have a more prominent role in interacting with lymph node immune cells and modulating adaptive immunity. The signals that direct neutrophils away from sites of inflammation in the periphery into lymphatic vessels and draining lymph nodes are yet to be fully understood. The two subpopulations of lymph node neutrophils, i.e., entering the lymph node from the circulation or inflamed tissues, may provide a possible explanation for the diverse effects of neutrophil depletion on the adaptive immune response
[48][49][123,124].
Inhibiting leukocyte trafficking is an effective strategy for treating a myriad of inflammatory diseases
[50][51][125,126]. Since tissue-egressing neutrophils and circulating neutrophils employ distinct molecular mechanisms and different routes of lymph node entry, it may be possible to specifically inhibit recruitment of one specific subpopulation. This suggests that treatment can be tailored to block one neutrophil subset but not the other and modulate a specific aspect of neutrophil interactions with the lymphatic system.
A less understood facet of neutrophil interactions with the lymphatic system is their ability to promote inflammatory lymphangiogenesis. Lymphangiogeneis has been associated with transplant rejection
[52][53][127,128] and inhibiting this process improves graft survival in corneal transplantation
[29][105]. However, the induction of therapeutic lymphangiogenesis mitigates allograft rejection in a orthotopic model of lung transplantation
[54][129]. In addition, preclinical studies in models of inflammatory skin disease
[43][55][117,130] demonstrated that promoting lymphangiogenesis may contribute to the resolution of inflammation. This suggests that neutrophil-driven stimulation of lymphangiogenesis may have two distinct and context-dependent roles in inflammation, i.e., promoting pathological inflammation or contributing to its resolution. The discovery of neutrophil-derived vascular endothelial factors involved in lymphangiogenesis presents an opportunity to develop targeted therapies for inflammatory disorders once these opposing roles are fully understood. Thus, clarifying neutrophil interactions with the lymphatic system is a crucial aspect in developing neutrophil-targeted interventions in inflammatory diseases.