Endophyte term literally means are 'inside plants'. Endophytes refer to fungal and bacterial organismscommunities that inhabit the plant endospheretissues of roots and shoots without showing any apparent symptoms nor harming their hosts.
- biotechnological applications
- endophytes
- plant growth
- biofertilizers
1. Introduction
Endophytes are fungal and bacterial corganismsmmunities that inhabit the plant endosphere without harmdamaging their hosts. They live asymptomatically in the plant cellular environment and perform symbiosis-specific functions such as synthesis of secondary metabolites or signaling molecules that function as internal and external stimuli during the mutualistic interaction [1]. Endophytic microbes are sources of novel biomolecules for the biochemical and pharmaceutical industries [2]. They produce biologically active metabolites, including immune-suppressive compounds, anticancer agents, plant growth promotors, antimicrobial volatiles, insecticides, antioxidants, and antibiotics [3][4], with huge potential for application in medicine, pharmaceutics industry, or agriculture (Figure 1). Moreover, endophytic microbes can improve plant growth under harsh conditions such as nutrient stress, temperature stress, salinity, trace metal stress, or drought [5]. They can also help plants to grow in contaminated environments by degrading hazardous compounds. We describe the main concepts of the application of endophytes in agriculture and biotechnology.
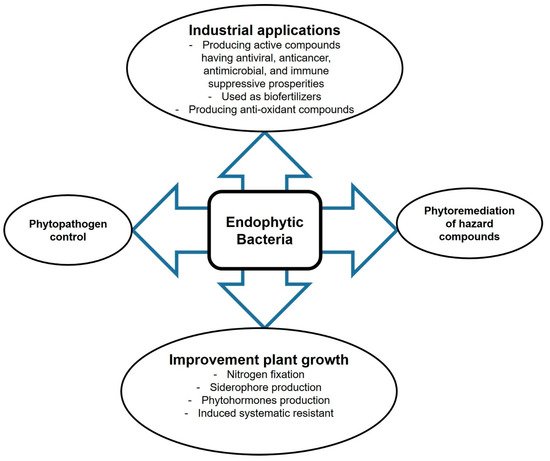
Figure 1. Prospective biotechnological applications of endophytic bacteria.
2. Types of Bacterial Endophytes
Taxonomically, endophytic bacteria belong to 16 phyla comprising more than 200 genera. However, the majority of them belong to the three phyla: Firmicutes, Actinobacteria, and Proteobacteria [6]. They are either Gram-negative or Gram-positive, such as Pseudomonas, Achromobacter, Agrobacterium, Xanthomonas, Acinetobacter, Microbacterium, Bacillus, and Brevibacterium [7]. Among others, mycoplasma has been isolated from the cytoplasm of marine green algae (e.g., Bryopsis hypnoides or Bryopsis pennata) [8].
Endophytic colonization can be local or systemic [9][10]. The plant endosphere represents a protective niche in which the endophytes are protected from biotic and abiotic stress. Moreover, endophytes can ecologically adapt to their environment and overcome plant defense responses [11].
Bacterial endophytes can also be classified as obligate or facultative endophytes. When endophytic bacteria rely on plant metabolites for survival and transfer between plants vertically or through the activity of different vectors, they are defined as obligate endophytes [12]. In comparison, facultative endophytes live outside the host at a definite stage of their life and are usually transmitted to plants from the surrounding atmosphere and soil [13].
3. Plant–Bacterial Endophyte Interactions
Interactions between bacteria and plants occur in many ways and at different levels (Figure 2). All plant organs interact with microorganisms at a specific stage of their life, and these interactions are not necessarily harmful to the plant. Plants can also benefit directly or indirectly from the interaction [14][15]. An example includes the well-studied rhizobia–legume interaction. Many endophytic bacteria form less specific symbiotic interactions with plants, although both partners adjust their metabolisms to the symbiotic conditions and can influence the biochemical properties of the partner [16]. This can result in promotion of the growth of the plant under normal and particularly harsh conditions [17][18].
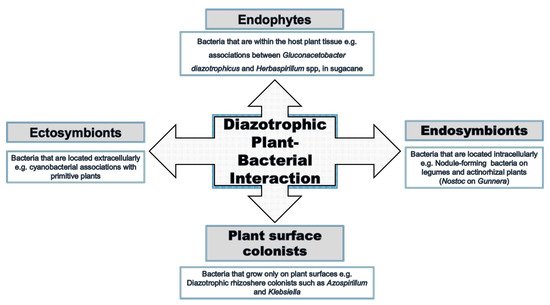
Figure 2. Endophytic plant–bacterial interactions versus other interactions.
Plants produce root exudates to attract beneficial bacteria, whereas bacterial endophytes recognize these compounds [19]. The bacteria in the root environment move towards the roots in response to the chemical attraction of the exudates [20]. After attachment to the root surface, they can enter the root, e.g., at lateral root emergence or openings caused by wounds or mechanical wounding [20]. Several bacterial structures are involved in their attachment to the plant surface, including fimbriae, flagella, bacterial surface polysaccharides, and lipopolysaccharides. For example, in Rhizobium spp., the surface polysaccharides are modified during the transition from free-living cells to the bacteroid form through the expression of surface antigens [21].
Bacterial entrance and spread inside plant tissues require plant cell wall modification, which is achieved by the secretion of cell-wall-degrading enzymes like endoglucanases, xylanases, cellulases, and pectinases by the endophytic bacteria [22][23]. Penetration into the host can be active or passive. Active penetration is completed by proliferation and attachment tools involving pili, flagella, twitching motility, and lipopolysaccharides, whereas quorum sensing influences bacterial colonization and movement in the host plant [24]. Passive penetration occurs through cracks on root tips or root emergence zones or arising from the activities of harmful organisms [12].
Bacterial colonization often begins at the root surface. After successful entry, the bacteria can move to aerial parts by the transpiration stream and with the support of the bacterial flagella [25]. This colonization pattern begins with intracellular microbial access through root hairs [26]. Endophytes can pass through the plant cell wall and enter the root cell either directly by the secretion of plant cell-wall-degrading enzymes and passage through the plant plasma membrane or by rhizophagy. Rhizophagy is a phenomenon in which many plants get microbes from the soil into their cells and digest them as a source of essential nutrients [27][28].
However, most endophytes reside in the intercellular spaces of their hosts, i.e., in sites that are rich in carbohydrates, inorganic nutrients, and amino acids [29]. Besides root colonization, endophytic bacteria can also occupy the intercellular spaces of stems, leaves, seeds, flowers, fruits, and xylem vessels [30][31][32][33][34]. Endophytes with intracellular colonization are difficult to study because they are often non-cultivable [35].
References
- Tidke, S.A.; Kiran, S.; Giridhar, P.; Gokare, R.A. Current Understanding and Future Perspectives of Endophytic Microbes vis-a-vis Production of Secondary Metabolites. In Endophytes and Secondary Metabolites; Jha, S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–16.
- Fouda, A.H.; Hassan, S.E.-D.; Eid, A.M.; Ewais, E.E.-D. Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.). Ann. Agric. Sci. 2015, 60, 95–104.
- Strobel, G. The Emergence of Endophytic Microbes and Their Biological Promise. J. Fungi 2018, 4, 57.
- Khalil, A.M.A.; Hassan, S.E.-D.; Alsharif, S.M.; Eid, A.M.; Ewais, E.E.-D.; Azab, E.; Gobouri, A.A.; Elkelish, A.; Fouda, A. Isolation and Characterization of Fungal Endophytes Isolated from Medicinal Plant Ephedra pachyclada as Plant Growth-Promoting. Biomolecules 2021, 11, 140.
- Eid, A.M.; Salim, S.S.; Hassan, S.E.-D.; Ismail, M.A.; Fouda, A. Role of Endophytes in Plant Health and Abiotic Stress Management. In Microbiome in Plant Health and Disease: Challenges and Opportunities; Kumar, V., Prasad, R., Kumar, M., Choudhary, D.K., Eds.; Springer: Singapore, 2019; pp. 119–144.
- Golinska, P.; Wypij, M.; Agarkar, G.; Rathod, D.; Dahm, H.; Rai, M. Endophytic actinobacteria of medicinal plants: Diversity and bioactivity. Antonie Van Leeuwenhoek 2015, 108, 267–289.
- Sun, H.; He, Y.; Xiao, Q.; Ye, R.; Tian, Y. Isolation, characterization, and antimicrobial activity of endophytic bacteria from Polygonum cuspidatum. Afr. J. Microbiol. Res. 2013, 7, 1496–1504.
- Hollants, J.; Leroux, O.; Leliaert, F.; Decleyre, H.; De Clerck, O.; Willems, A. Who Is in There? Exploration of Endophytic Bacteria within the Siphonous Green Seaweed Bryopsis (Bryopsidales, Chlorophyta). PLoS ONE 2011, 6, e26458.
- Rangjaroen, C.; Sungthong, R.; Rerkasem, B.; Teaumroong, N.; Noisangiam, R.; Lumyong, S. Untapped Endophytic Colonization and Plant Growth-Promoting Potential of the Genus Novosphingobium to Optimize Rice Cultivation. Microbes Environ. 2017, 32, 84–87.
- Castanheira, N.L.; Dourado, A.C.; Pais, I.; Semedo, J.; Scotti-Campos, P.; Borges, N.; Carvalho, G.; Crespo, M.T.B.; Fareleira, P. Colonization and beneficial effects on annual ryegrass by mixed inoculation with plant growth promoting bacteria. Microbiol. Res. 2017, 198, 47–55.
- Mercado-Blanco, J.; Lugtenberg, B.J.J. Biotechnological Applications of Bacterial Endophytes. Curr. Biotechnol. 2014, 3, 60–75.
- Hardoim, P.R.; van Overbeek, L.S.; Elsas, J.D.v. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471.
- Abreu-Tarazi, M.F.; Navarrete, A.A.; Andreote, F.D.; Almeida, C.V.; Tsai, S.M.; Almeida, M. Endophytic bacteria in long-term in vitro cultivated “axenic” pineapple microplants revealed by PCR–DGGE. World J. Microbiol. Biotechnol. 2010, 26, 555–560.
- Schirawski, J.; Perlin, M.H. Plant–Microbe Interaction 2017—The Good, the Bad and the Diverse. Int. J. Mol. Sci. 2018, 19, 1374.
- Hassan, S.E.-D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J Adv Res 2017, 8, 687–695.
- Doty, S.L. Symbiotic Plant-Bacterial Endospheric Interactions. Microorganisms 2018, 6, 28.
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49.
- Nair, D.N.; Padmavathy, S. Impact of Endophytic Microorganisms on Plants, Environment and Humans. Sci. World J. 2014, 2014, 250693.
- Rosenblueth, M.; Martínez-Romero, E. Bacterial Endophytes and Their Interactions with Hosts. Mol. Plant-Microbe Interact. 2006, 19, 827–837.
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial Endophyte Colonization and Distribution within Plants. Microorganisms 2017, 5.
- Balsanelli, E.; Serrato, R.V.; De Baura, V.A.; Sassaki, G.; Yates, M.G.; Rigo, L.U.; Pedrosa, F.O.; De Souza, E.M.; Monteiro, R.A. Herbaspirillum seropedicae rfbB and rfbC genes are required for maize colonization. Environ. Microbiol. 2010, 12, 2233–2244.
- Reinhold-Hurek, B.; Maes, T.; Gemmer, S.; Van Montagu, M.; Hurek, T. An Endoglucanase Is Involved in Infection of Rice Roots by the Not-Cellulose-Metabolizing Endophyte Azoarcus Sp. Strain BH72. Mol. Plant-Microbe Interact. 2006, 19, 181–188.
- Fouda, A.; Abdel-Maksoud, G.; Saad, H.A.; Gobouri, A.A.; Mohammedsaleh, Z.M.; El-Sadany, M.A. The efficacy of silver nitrate (AgNO3) as a coating agentto protect paper against high deteriorating microbes. Catalysts 2021, 11, 310.
- Suárez-Moreno, Z.R.; Devescovi, G.; Myers, M.; Hallack, L.; Mendonça-Previato, L.; Caballero-Mellado, J.; Venturi, V. Commonalities and Differences in Regulation of N-Acyl Homoserine Lactone Quorum Sensing in the Beneficial Plant-Associated Burkholderia Species Cluster. Appl. Environ. Microbiol. 2010, 76, 4302.
- Compant, S.; Reiter, B.; Sessitsch, A.; Nowak, J.; Clément, C.; Ait Barka, E. Endophytic Colonization of Vitis vinifera L. by Plant Growth-Promoting Bacterium Burkholderia sp. Strain PsJN. Appl. Environ. Microbiol. 2005, 71, 1685.
- Prieto, P.; Schilirò, E.; Maldonado-González, M.M.; Valderrama, R.; Barroso-Albarracín, J.B.; Mercado-Blanco, J. Root hairs play a key role in the endophytic colonization of olive roots by Pseudomonas spp. with biocontrol activity. Microb. Ecol. 2011, 62, 435–445.
- White, J.F., Jr.; Torres, M.S.; Somu, M.P.; Johnson, H.; Irizarry, I.; Chen, Q.; Zhang, N.; Walsh, E.; Tadych, M.; Bergen, M. Hydrogen peroxide staining to visualize intracellular bacterial infections of seedling root cells. Microsc. Res. Tech. 2014, 77, 566–573.
- Paungfoo-Lonhienne, C.; Rentsch, D.; Robatzek, S.; Webb, R.I.; Sagulenko, E.; Näsholm, T.; Schmidt, S.; Lonhienne, T.G.A. Turning the Table: Plants Consume Microbes as a Source of Nutrients. PLoS ONE 2010, 5, e11915.
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293.
- Kandel, S.L.; Herschberger, N.; Kim, S.H.; Doty, S.L. Diazotrophic Endophytes of Poplar and Willow for Growth Promotion of Rice Plants in Nitrogen-Limited Conditions. Crop Sci. 2015, 55, 1765–1772.
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959.
- Mitter, B.; Pfaffenbichler, N.; Flavell, R.; Compant, S.; Antonielli, L.; Petric, A.; Berninger, T.; Naveed, M.; Sheibani-Tezerji, R.; von Maltzahn, G.; et al. A New Approach to Modify Plant Microbiomes and Traits by Introducing Beneficial Bacteria at Flowering into Progeny Seeds. Front. Microbiol. 2017, 8, 11.
- Glassner, H.; Zchori-Fein, E.; Yaron, S.; Sessitsch, A.; Sauer, U.; Compant, S. Bacterial niches inside seeds of Cucumis melo L. Plant Soil 2018, 422, 101–113.
- Koskimäki, J.J.; Pirttilä, A.M.; Ihantola, E.-L.; Halonen, O.; Frank, A.C. The Intracellular Scots Pine Shoot Symbiont Methylobacterium extorquens DSM13060 Aggregates around the Host Nucleus and Encodes Eukaryote-Like Proteins. mBio 2015, 6, e00039-15.
- Thomas, P.; Reddy, K.M. Microscopic elucidation of abundant endophytic bacteria colonizing the cell wall–plasma membrane peri-space in the shoot-tip tissue of banana. AOB Plants 2013, 5.
