Integrin β4 (ITGβ4) is a class of transmembrane adhesion molecules composed of hemidesmosomes (HDs). Its unique long intracellular domain provides intricate signal transduction functions. These signal transduction effects are especially prominent in tumors. Integrin β4 is differentially expressed in various tumors, and it plays a vital role in tumor invasion, proliferation, epithelial–mesenchymal transition, and angiogenesis. In clinical practice, it is described as a diagnostic marker for the targeted treatment of cancer and will be helpful in the clinical diagnosis and treatment of tumors.
- integrin β4
- cancer
- diagnostic significance
1. Function of Integrin β4
[1]
[2]
[3]
[4]
[2]
[4]
[5]
[6]
2. Differential Expression of Integrin β4 in Tumors
2.1. Lung Cancer
[7]
[10]
2.2. Breast Cancer
[11]
[12]
[13]
[14]
[14]
[15]
[16]
[17]
[18]
2.3. Prostate Cancer
[19]
[20]
[21]
2.4. Colon Cancer
[17]
[22]
[23]
[24]
[25]
[26]
Table 1
Table 1.
| Lung Cancer | Breast Cancer | Prostate Cancer | Colon Cancer |
|---|
| Expression Level | ① Abundantly present ② Strongly overexpressed |
① Occurs in transitional breast cancer cells ② Not in non-invasive breast cancer cells |
Overexpressed (has a large CpG island) | ① Wild-type form is increased ② Integrin β4A(ctd-) is predominantly absent |
| Function | ① Initiates phosphorylation, invasion, and migration ② Occurs with gene mutation |
① Induces invasive status ② Enhances cell viability and motility ③ Promotes angiogenesis |
① Induces tumorigenesis ② Induces cell migration and invasion ③ Does not affect cell proliferation |
① Induces abnormal cell proliferation ② Affects the cell viability ③ Induces metastasis |
3. Integrin-β4-Mediated Cancer Progression
[10]
[27]
[10]
3.1. Integrin β4 Is Associated with Tumor Invasion and Migration
[28]
[29]
[35]
[39]
[40]
[5]
[41]
[40]
[41]
Figure 1
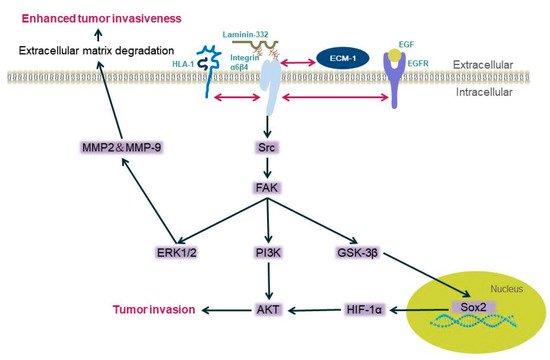
Figure 1.
[6]
2+
[46]
[17]
[47]
[48]
[49]
[49]
3.2. Integrin β4 Is Associated with Tumor Cell Proliferation
[51]
[52]
[15]
3.3. Integrin β4 Is Related to Epithelial–Mesenchymal Transition
[53]
[56]
[57]
[62]
[63]
[41]
[64]
3.4. Integrin β4 Is Associated with Angiogenesis
[67]
[18]
[68]
[67].
References
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215.
- Chi, Y.X.; Xiang, Y.; Qin, X.Q. The research progress of integrin beta4. Sheng Li Xue Bao 2018, 70, 504–510.
- Kariya, Y.; Gu, J. Roles of Integrin alpha6beta4 Glycosylation in Cancer. Cancers 2017, 9, 79.
- Miyazaki, K. Laminin-5 (laminin-332): Unique biological activity and role in tumor growth and invasion. Cancer Sci. 2006, 97, 91–98.
- Margadant, C.; Frijns, E.; Wilhelmsen, K.; Sonnenberg, A. Regulation of hemidesmosome disassembly by growth factor receptors. Curr. Opin. Cell Biol. 2008, 20, 589–596.
- Kariya, Y.; Oyama, M.; Hashimoto, Y.; Gu, J.; Kariya, Y. beta4-Integrin/PI3K Signaling Promotes Tumor Progression through the Galectin-3-N-Glycan Complex. Mol. Cancer Res. 2018, 16, 1024–1034.
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, Mucus, and Goblet Cells. Chest 2018, 154, 169–176.
- Yen, C.H.; Li, S.T.; Cheng, N.C.; Ji, Y.R.; Wang, J.H.; Young, T.H. Label-free platform on pH-responsive chitosan: Adhesive heterogeneity for cancer stem-like cell isolation from A549 cells via integrin beta4. Carbohydr. Polym. 2020, 239, 116168.
- Lakshmanan, I.; Rachagani, S.; Hauke, R.; Krishn, S.R.; Paknikar, S.; Seshacharyulu, P.; Karmakar, S.; Nimmakayala, R.K.; Kaushik, G.; Johansson, S.L.; et al. MUC5AC interactions with integrin beta4 enhances the migration of lung cancer cells through FAK signaling. Oncogene 2016, 35, 4112–4121.
- Stewart, R.L.; West, D.; Wang, C.; Weiss, H.L.; Gal, T.; Durbin, E.B.; O’Connor, W.; Chen, M.; O’Connor, K.L. Elevated integrin alpha6beta4 expression is associated with venous invasion and decreased overall survival in non-small cell lung cancer. Hum. Pathol. 2016, 54, 174–183.
- Soung, Y.H.; Ford, S.; Yan, C.; Chung, J. The Role of Arrestin Domain-Containing 3 in Regulating Endocytic Recycling and Extracellular Vesicle Sorting of Integrin beta4 in Breast Cancer. Cancers 2018, 10, 507.
- Li, J.; Sun, H.; Feltri, M.L.; Mercurio, A.M. Integrin beta4 regulation of PTHrP underlies its contribution to mammary gland development. Dev. Biol. 2015, 407, 313–320.
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335.
- Bierie, B.; Pierce, S.E.; Kroeger, C.; Stover, D.G.; Pattabiraman, D.R.; Thiru, P.; Donaher, J.L.; Reinhardt, F.; Chaffer, C.L.; Keckesova, Z.; et al. Integrin-beta4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc. Natl. Acad. Sci. USA 2017, 114, E2337–E2346.
- Sung, J.S.; Kang, C.W.; Kang, S.; Jang, Y.; Chae, Y.C.; Gil Kim, B.; Cho, N.H. ITGB4-mediated metabolic reprogramming of cancer-associated fibroblasts. Oncogene 2020, 39, 664–676.
- Coleman, D.T.; Soung, Y.H.; Surh, Y.J.; Cardelli, J.A.; Chung, J. Curcumin Prevents Palmitoylation of Integrin beta4 in Breast Cancer Cells. PLoS ONE 2015, 10, e0125399.
- Ho, J.Y.; Chang, F.W.; Huang, F.S.; Liu, J.M.; Liu, Y.P.; Chen, S.P.; Liu, Y.L.; Cheng, K.C.; Yu, C.P.; Hsu, R.J. Estrogen Enhances the Cell Viability and Motility of Breast Cancer Cells through the ERalpha-DeltaNp63-Integrin beta4 Signaling Pathway. PLoS ONE 2016, 11, e0148301.
- Siddharth, S.; Nayak, A.; Das, S.; Nayak, D.; Panda, J.; Wyatt, M.D.; Kundu, C.N. The soluble nectin-4 ecto-domain promotes breast cancer induced angiogenesis via endothelial Integrin-beta4. Int. J. Biochem. Cell Biol. 2018, 102, 151–160.
- Yoshioka, T.; Otero, J.; Chen, Y.; Kim, Y.M.; Koutcher, J.A.; Satagopan, J.; Reuter, V.; Carver, B.; De Stanchina, E.; Enomoto, K.; et al. beta4 Integrin signaling induces expansion of prostate tumor progenitors. J. Clin. Investig. 2013, 123, 682–699.
- Wilkinson, E.J.; Woodworth, A.M.; Parker, M.; Phillips, J.L.; Malley, R.C.; Dickinson, J.L.; Holloway, A.F. Epigenetic regulation of the ITGB4 gene in prostate cancer. Exp. Cell Res. 2020, 392, 112055.
- Kawakami, K.; Fujita, Y.; Kato, T.; Mizutani, K.; Kameyama, K.; Tsumoto, H.; Miura, Y.; Deguchi, T.; Ito, M. Integrin beta4 and vinculin contained in exosomes are potential markers for progression of prostate cancer asso-ciated with taxane-resistance. Int. J. Oncol. 2015, 47, 384–390.
- Basora, N.; Herring-Gillam, F.E.; Boudreau, F.; Perreault, N.; Pageot, L.P.; Simoneau, M.; Bouatrouss, Y.; Beaulieu, J.F. Expression of functionally distinct variants of the beta(4)A integrin subunit in relation to the differentiation state in human intestinal cells. J. Biol. Chem. 1999, 274, 29819–29825.
- Ni, H.; Dydensborg, A.B.; Herring, F.E.; Basora, N.; Gagné, D.; Vachon, P.H.; Beaulieu, J.-F. Upregulation of a functional form of the beta4 integrin subunit in colorectal cancers correlates with c-Myc expression. Oncogene 2005, 24, 6820–6829.
- Tanaka, N.; Ohno, Y.; Nobuhisa, T.; Takaoka, M.; Sirmali, M.; Nakajima, M.; Gunduz, M.; Shirakawa, Y.; Okawa, T.; Naomoto, Y.; et al. Localization of FAK is related with colorectal carcinogenesis. Int. J. Oncol. 2008, 32, 791–796.
- Lü, Y.; Han, B.; Yu, H.; Cui, Z.; Li, Z.; Wang, J. Berberine regulates the microRNA-21-ITGBeta4-PDCD4 axis and inhibits colon cancer viability. Oncol. Lett. 2018, 15, 5971–5976.
- Uemura, T.; Shiozaki, K.; Yamaguchi, K.; Miyazaki, S.; Satomi, S.; Kato, K.; Sakuraba, H.; Miyagi, T. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene 2009, 28, 1218–1229.
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635.
- Draheim, K.M.; Chen, H.B.; Tao, Q.; Moore, N.; Roche, M.; Lyle, S. ARRDC3 suppresses breast cancer progression by negatively regulating integrin beta4. Oncogene 2010, 29, 5032–5047.
- Elaimy, A.L.; Wang, M.; Sheel, A.; Brown, C.W.; Walker, M.R.; Amante, J.J.; Xue, W.; Chan, A.; Baer, C.E.; Goel, H.L.; et al. Real-time imaging of integrin beta4 dynamics using a reporter cell line generated by Crispr/Cas9 genome editing. J. Cell. Sci. 2019, 132, jcs231241.
- Lotz, M.M.; Nusrat, A.; Madara, J.L.; Ezzell, R.; Wewer, U.M.; Mercurio, A.M. Intestinal epithelial restitution. Involvement of specific laminin isoforms and integrin laminin receptors in wound closure of a transformed model epithelium. Am. J. Pathol. 1997, 150, 747–760.
- O’Connor, K.L.; Nguyen, B.K.; Mercurio, A.M. RhoA function in lamellae formation and migration is regulated by the alpha6beta4 integrin and cAMP metabolism. J. Cell Biol. 2000, 148, 253–258.
- Rabinovitz, I.; Mercurio, A.M. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J. Cell Biol. 1997, 139, 1873–1884.
- Rabinovitz, I.; Toker, A.; Mercurio, A.M. Protein kinase C-dependent mobilization of the alpha6beta4 integrin from hemidesmo-somes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J. Cell Biol. 1999, 146, 1147–1160.
- Shaw, L.M.; Rabinovitz, I.; Wang, H.H.; Toker, A.; Mercurio, A.M. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell 1997, 91, 949–960.
- O’Connor, K.; Chen, M. Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases 2013, 4, 141–147.
- Lipscomb, E.A.; Mercurio, A.M. Mobilization and activation of a signaling competent alpha6beta4integrin underlies its contribution to carcinoma progression. Cancer Metastasis Rev. 2005, 24, 413–423.
- Nikolopoulos, S.N.; Blaikie, P.; Yoshioka, T.; Guo, W.; Puri, C.; Tacchetti, C.; Giancotti, F.G. Targeted deletion of the integrin beta4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-kappaB, causing defects in epidermal growth and migration. Mol. Cell Biol. 2005, 25, 6090–6102.
- Yoon, S.O.; Shin, S.; Lipscomb, E.A. A novel mechanism for integrin-mediated ras activation in breast carcinoma cells: The al-pha6beta4 integrin regulates ErbB2 translation and transactivates epidermal growth factor receptor/ErbB2 signaling. Cancer Res. 2006, 66, 2732–2739.
- Li, J.; Luo, M.; Ou, H.; Liu, X.; Kang, X.; Yin, W. Integrin beta4 promotes invasion and anoikis resistance of papillary thyroid carcinoma and is consistently overexpressed in lymphovascular tumor thrombus. J. Cancer 2019, 10, 6635–6648.
- Zhang, X.; Rozengurt, E.; Reed, E.F. HLA class I molecules partner with integrin beta4 to stimulate endothelial cell proliferation and migration. Sci. Signal 2010, 3, ra85.
- Gan, L.; Meng, J.; Xu, M.; Liu, M.; Qi, Y.; Tan, C.; Wang, Y.; Zhang, P.; Weng, W.; Sheng, W.; et al. Extracellular matrix protein 1 promotes cell metastasis and glucose metabolism by inducing integrin be-ta4/FAK/SOX2/HIF-1alpha signaling pathway in gastric cancer. Oncogene 2018, 37, 744–755.
- Leng, C.; Zhang, Z.-G.; Chen, W.-X.; Luo, H.-P.; Song, J.; Dong, W.; Zhu, X.-R.; Chen, X.-P.; Liang, H.-F.; Zhang, B.-X. An integrin beta4-EGFR unit promotes hepatocellular carcinoma lung metastases by enhancing anchorage independence through activation of FAK–AKT pathway. Cancer Lett. 2016, 376, 188–196.
- Tai, Y.L.; Chu, P.Y.; Lai, I.R.; Wang, M.Y.; Tseng, H.Y.; Guan, J.L.; Liou, J.Y.; Shen, T.L. An EGFR/Src-dependent beta4 integrin/FAK complex contributes to malignancy of breast cancer. Sci. Rep. 2015, 5, 16408.
- Zhu, J.; Wu, Y.-N.; Zhang, W.; Zhang, X.-M.; Ding, X.; Li, H.-Q.; Geng, M.; Xie, Z.-Q.; Wu, H.-M. Monocarboxylate Transporter 4 Facilitates Cell Proliferation and Migration and Is Associated with Poor Prognosis in Oral Squamous Cell Carcinoma Patients. PLoS ONE 2014, 9, e87904.
- Marchese, V.; Juarez, J.; Patel, P.; Hutter-Lobo, D. Density-dependent ERK MAPK expression regulates MMP-9 and influences growth. Mol. Cell. Biochem. 2019, 456, 115–122.
- Jeon, J.H.; Suh, H.N.; Kim, M.O.; Han, H.J. Glucosamine-induced reduction of integrin beta4 and plectin complex stimulates migration and proliferation in mouse embryonic stem cells. Stem. Cells Dev. 2013, 22, 2975–2989.
- Gerson, K.D.; Shearstone, J.R.; Maddula, V.K.; Seligmann, B.E.; Mercurio, A.M. Integrin beta4 regulates SPARC protein to promote invasion. J. Biol. Chem. 2012, 287, 9835–9844.
- Stewart, R.L.; O’Connor, K.L. Clinical significance of the integrin alpha6beta4 in human malignancies. Lab. Investig. 2015, 95, 976–986.
- Te Molder, L.; Sonnenberg, A. PKD2 and RSK1 Regulate Integrin beta4 Phosphorylation at Threonine 1736. PLoS ONE 2015, 10, e0143357.
- Chen, W.; Sammani, S.; Mitra, S.; Ma, S.F.; Garcia, J.G.; Jacobson, J.R. Critical role for integrin-beta4 in the attenuation of murine acute lung injury by simvastatin. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L279–L285.
- Cirulli, V.; Yebra, M. Netrins: Beyond the brain. Nat. Rev. Mol. Cell Biol. 2007, 8, 296–306.
- Hu, Y.; Ylivinkka, I.; Chen, P.; Li, L.; Hautaniemi, S.; Nyman, T.A.; Keski-Oja, J.; Hyytiäinen, M. Netrin-4 promotes glioblastoma cell proliferation through integrin beta4 signaling. Neoplasia 2012, 14, 219–227.
- Chang, C.; Yang, X.; Pursell, B.; Mercurio, A.M. Id2 complexes with the SNAG domain of Snai1 inhibiting Snai1-mediated repression of integrin beta4. Mol. Cell Biol. 2013, 33, 3795–3804.
- Hugo, H.; Ackland, L.; Blick, T.; Lawrence, M.G.; Clements, J.; Williams, E.D.; Thompson, E.W. Epithelial—mesenchymal and mesenchymal—epithelial transitions in carcinoma progression. J. Cell. Physiol. 2007, 213, 374–383.
- Farahani, E.; Patra, H.K.; Jangamreddy, J.R.; Rashedi, I.; Kawalec, M.; Pariti, R.K.R.; Batakis, P.; Wiechec, E. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinog. 2014, 35, 747–759.
- Chen, X.F.; Zhang, H.J.; Wang, H.B.; Zhu, J.; Zhou, W.Y.; Zhang, H.; Zhao, M.C.; Su, J.M.; Gao, W.; Zhang, L. Transforming growth factor-beta1 induces epithelial-to-mesenchymal transition in human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling pathways. Mol. Biol. Rep. 2012, 39, 3549–3556.
- Wang, Q.; Wang, Y.; Huang, X.; Liang, W.; Xiong, Z.; Xiong, Z. Integrin beta4 in EMT: An implication of renal diseases. Int. J. Clin. Exp. Med. 2015, 8, 6967–6976.
- Liu, H.; Radisky, D.C.; Yang, D.; Xu, R.; Radisky, E.S.; Bissell, M.J.; Bishop, J.M. MYC suppresses cancer metastasis by direct transcriptional silencing of alphav and beta3 integrin subunits. Nat. Cell. Biol. 2012, 14, 567–574.
- Giannelli, G.; Villa, E.; Lahn, M. Targeting transforming growth factor (TGF)-betaRI inhibits activation of beta1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology 2009, 49, 839–850.
- Yeh, Y.Y.; Chiao, C.C.; Kuo, W.Y.; Hsiao, Y.C.; Chen, Y.J.; Wei, Y.Y. TGF-beta1 increases motility and alphavbeta3 integrin up-regulation via PI3K, Akt and NF-kappaB-dependent pathway in human chondrosarcoma cells. Biochem. Pharmacol. 2008, 75, 1292–1301.
- Zhang, Y.J.; Tian, Z.L.; Yu, X.Y.; Zhao, X.X.; Yao, L. Activation of integrin beta1-focal adhesion kinase-RasGTP pathway plays a critical role in TGF beta1-induced po-docyte injury. Cell Signal. 2013, 25, 2769–2779.
- Takaoka, A.S.; Yamada, T.; Gotoh, M.; Kanai, Y.; Imai, K.; Hirohashi, S. Cloning and characterization of the human beta4-integrin gene promoter and enhancers. J. Biol. Chem. 1998, 273, 33848–33855.
- Yang, X.; Pursell, B.; Lu, S.; Chang, T.K.; Mercurio, A.M. Regulation of beta 4-integrin expression by epigenetic modifications in the mammary gland and during the epitheli-al-to-mesenchymal transition. J. Cell Sci. 2009, 122, 2473–2480.
- Li, J.; Hao, N.; Han, J.; Zhang, M.; Li, X.; Yang, N. ZKSCAN3 drives tumor metastasis via integrin beta4/FAK/AKT mediated epithelial-mesenchymal transition in hepato-cellular carcinoma. Cancer Cell. Int. 2020, 20, 216.
- Bergers, G.; Hanahan, D.; Coussens, L.M. Angiogenesis and apoptosis are cellular parameters of neoplastic progression in transgenic mouse models of tumorigenesis. Int. J. Dev. Biol. 1998, 42, 995–1002.
- Kaur, B.; Khwaja, F.W.; Severson, E.A.; Matheny, S.L.; Brat, D.J.; Van Meir, E.G. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-Oncology 2005, 7, 134–153.
- Nikolopoulos, S.N.; Blaikie, P.; Yoshioka, T.; Guo, W.; Giancotti, F.G. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell 2004, 6, 471–483.
- Ephstein, Y.; Singleton, P.A.; Chen, W.; Wang, L.; Salgia, R.; Kanteti, P.; Dudek, S.M.; Garcia, J.G.; Jacobson, J.R. Critical role of S1PR1 and integrin beta4 in HGF/c-Met-mediated increases in vascular integrity. J. Biol. Chem. 2013, 288, 2191–2200.
