Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Muhammad Humayun and Version 2 by Lindsay Dong.
Cobalt catalysts are very important due to their extensive applications in many industrial processes, such as Fisher–Tropsh synthesis [82,83] and CO2 conversion [84]. Electrocatalytic CO2 reduction reaction (CO2RR) is a promising strategy due to its easy operating system, simple constructions, operational at neutral pH, ambient temperature and atmospheric pressure, and low energy utilization to produce valuable chemicals and fuels such as formic acid, methane, ethanol, and carbon using renewable electricity. Therefore, CO2RR coupling with renewable energy sources can effectively achieve a carbon-neutral energy cycle and hydrocarbon products with high activity, stability, and selectivity [85,86].
- CO2 conversion
- electrocatalysts
- cobalt catalysts
- MOFs
1. Introduction
The excessive combustion of fossil fuels has caused massive carbon dioxide (CO2) emissions, leading to rapid global environmental changes such as global warming, air pollution, desertification, acid rains, rise in sea levels, and extreme weather conditions [1]. The threats to human life and the environment due to high CO2 emissions are increasing day by day with growing energy demands.
Several carbon capture and utilization methods are implemented to mitigate CO2 concentration in the atmosphere and overcome its environmental challenges [2][3][4][5][6][4,5,6,7,8]. The main strategies to reduce CO2 emissions deal with the circular carbon economy (CCE), a holistic approach that consists of Reduce, Reuse, Recycle and Remove (4Rs) of CO2. The reuse of CO2 is categorized to search for low carbon energy alternatives such as wind, solar and hydro energy for replacing fossil fuels. Another approach is geological sequestration, a promising strategy to provide a low carbon energy future [7][8][9][9,10,11]. Still, there is uncertainty about stored CO2 for a long time, and it might have leakage issues. Another approach is recycling, and utilizing CO2 into other useful chemicals is the most attractive strategy to reduce CO2 emissions [10][11][12][12,13,14]. Catalysis plays a vital role in our daily life. Various types of catalysts have been reported for the conversion of waste into useful products, including zeolites [13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][15,16,17,18,19,20,21,22,23,24,25,26,27,28,29], metal and metal oxides [28][29][30][31][32][33][34][35][36][37][38][39][40][30,31,32,33,34,35,36,37,38,39,40,41,42], nitrides [41][42][43][44][45][46][47][48][49][43,44,45,46,47,48,49,50,51], carbon-based catalysts [50][51][52][53][54][55][56][57][58][59][60][61][52,53,54,55,56,57,58,59,60,61,62,63], metal complexes [62][63][64][65][66][67][68][69][70][64,65,66,67,68,69,70,71,72] or highly porous metal–organic frameworks (MOFs) and covalent organic frameworks (COFs) [3][71][72][73][74][75][76][77][78][5,73,74,75,76,77,78,79,80]. The synthesis of supported catalysts methods has also been reviewed recently [79][81]. We observed the superior behavior of cobalt in catalysis, especially in electrocatalysis.
Co-based materials have many advantages over others because as a popular metal, Co belongs to the group VIII-B of the periodic table having unique features like high electrical conductivity, thermal stability, unique electronic features, chemical stability, and high catalytic performances, which makes co-based catalysts as promising materials for CO2RR applications. Cobalt as an earth-abundant transition metal is a splendid alternative to noble metals such as Pt, Ir, Ru, etc. For CO2RR, Co has been used as a prominent source as noble metal-free electro/photocatalysts due to fascinating properties such as loosely bonded d-electrons and therefore readily available multiple oxidation states (Co(0), Co(I), Co(II), Co(III) and Co(IV). Moreover, it is found that a transition from Co(II) to Co(I) is involved at the intermediate state for CO2 reduction. Hence, high activity, outstanding stability and product selectivity are achieved through Co-based catalysts for CO2 reduction [80][81][84,102]. Cobalt is more reactive than other earth-abundant metals due to the possession of modest CO2 adsorption and d-band closeness to the Fermi level [82][103]. Co-based catalysts have been explored as effective cathode materials for electroreduction of CO2 to CO exhibiting high activities and selectivity [83][104]. For CO2 photoreduction, Co metal sites in Co-based MOFs offer the traps for electrons for facilitation in electrons-holes separation, thus providing a longer life for electrons for the reduction reaction. Co is found to be an important stabilizer for major intermediates in CO2 reduction [80][82][84,103]. Co-based materials have applications in various other fields such as energy storage, catalysis, and thermopower. Co-based materials (i.e., NaxCoO2) play a critical role in cathode and anode materials for Na-ion batteries. Likewise, LiCoO2 has been regarded as one of the most commercialized cathode materials for Li-ion batteries. Cobalt oxides and cobalt chalcogenides exhibit a high theoretical capacity for sodium storage [84][105]. Thus, cobalt has been reported as an important center for CO2 reduction [38][44][85][86][87][88][40,46,106,107,108,109].
2. Cobalt Catalysts for CO2 Reduction
2.1. Cobalt-Based Single-Atom Catalysts (SACs) for CO
2
RR
CO2 reduction reaction (CO2RR) catalysts have challenges of high overpotential, low Faradaic efficiency, low current density and lack of long-term stability. However, single-atom catalysts (SACs) are used for CO2RR with great importance. Studies show that SACs for CO2RR are of two categories based on their synthesis route: i) organometallic precursor pyrolysis such as MOFs; ii) loading of the metal precursor directly onto the support, which are followed by heat and acids treatment to get rid of the excess nanoparticles as shown in Figure 12. The Co precursor was dispersed with the polymer (Pluronic F127) and the colloidal silica, then the mixture was pyrolyzed and the template was etched by acid treatment to obtain the Co-SAS/HOPNC. Uniform hierarchical and atomic sites of cobalt dispersed in the carbon matrix were observed by the BET. SEM and TEM and the elemental analysis confirm the presence of the Co, N and C.
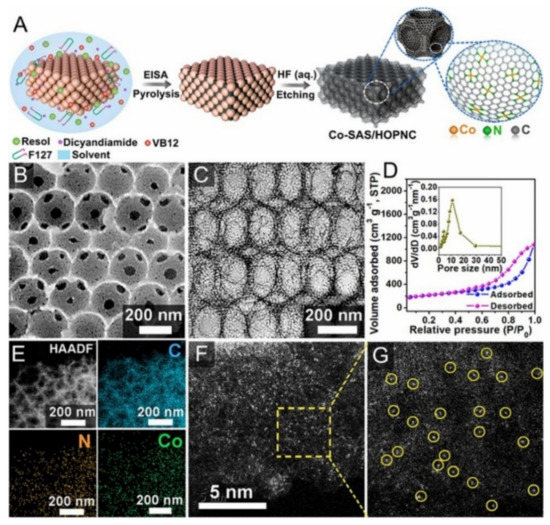

Figure 12. Fabrication of Co-SAS/HOPNC catalyst. (A) Step-wise synthesis procedure of Co-SAS/HOPNC. (B) SEM image, and (C) TEM micrograph of Co-SAS/HOPNC catalyst. (D) N2 adsorption–desorption isotherm curves of Co-SAS/HOPNC catalyst. (Inset) The pore size distribution curve. (E) HAADF-STEM micrograph and the EDS maps of Co-SAS/HOPNC catalyst. (F,G) AC HAADF-STEM micrographs of the Co-SAS/HOPNC catalyst. Yellow circles represent the isolated single Co atoms. Reproduced from [89][119], with permission from PNAS, 2018.
2.2. Multi-Metals Cobalt Catalysts for CO
2
RR
Electrochemical CO2RR produces various forms of products ranging from CO, formic acid, alcohols, methane, olefin, and hydrocarbon [90][123]. However, the selectivity of these products is highly dependent on the adsorption characteristics of the reactants on the electrode surface. Due to the associated problems of metal electrocatalyst, transition metal catalysts could solve the problems to some extent because of the overpotential and poisoning [91][124], as metal and mixed metal electro catalysis still attracted scientific attention [92][93][125,126]. Recently, multimetallic compounds have been given more attention to reducing material cost and tuning the strength of intermediate electrochemical reaction of CO2 reduction to achieve high selectivity [94][127]. Multi-metals Co and Fe electrocatalyst were investigated for CO2-reduction by Abdinejad and coworkers [69][95][71,99]. Their research finding reveals that the introduction of amino substituent enhanced the electrolytic activity toward the CO2 conversion through dual active sites. The mono-amino FeP reduces the CO2 to CO at ambient temperature and pressure with significant turnover (TON). They further reported the reactivity and selectivity of amino compounds towards capture and electroreduction of CO2 in both homogeneous and heterogeneous [96][128].
2.3. Cobalt Oxides Catalysts for CO
2
RR
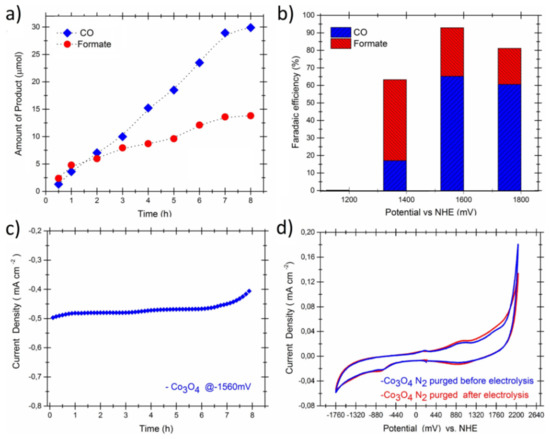
Cobalt oxides have been used extensively in CO2RR as active catalysts as well as support materials. Aljabour et al. [97][131] used nanofibrous Co3O4 for the production of CO and formic acid. The nanofiber electrode exhibits stability for 8 h and overall Faradaic efficiency of about 90% for CO at a geometric current density of ~0.5 mAcm−2 on a flat surface shown in Figure 25.
Figure 25. Electroreduction of CO2 via the nanofibrous Co3O4, (a) increase in the amount of CO gas and formate as a function of time at a regular electrolysis potential of −1560 mV vs. NHE, (b) the electrolysis voltage versus the faradaic efficiency, (c) the chronoamperometry results, (d) Cyclic voltammograms of the electrode nanofibers measured before and after the electrolysis at a scan rate of 30 mV s−1. Reproduced from [97][131], with permission from Elsevier, 2018.
2.4. Cobalt-Based Nitride Catalysts
Cobalt nitrides have proved beneficial for CO2RR because they offer more surface base sites of adsorption of CO2 and the generation of more active sites [98][99][100][101][102][103][104][140,141,142,143,144,145,146]. Peng et al. reported the highly efficient CO2RR cobalt nitride catalysts (700-Co5.47N/C) in an aqueous electrolyte. For the synthesis of these cobalt nitride catalysts, impregnation and nitridation involving temperature-programmed reaction (TRP) were used. For the optimized electrocatalyst 700-Co5.47N/C, the observed CO current density was 9.78 mA. cm−2 at −0.7 V vs. RHE. The as-prepared electrocatalyst showed high Faradaic efficiency and good stability. Furthermore, tuning of the electrolysis potentials led to the CO/H2 ratio adjustment from 3:1 to 3:2 [42][44]. In 2019, a robust synthesis strategy was adopted to synthesize the metal/nitrogen/carbon (MNC) catalysts with the presence of metal atoms as the atomically dispersed metal-Nx moieties (wherein MNx, M represents Mn, Co, Fe, Ni, and Cu metals) in N doped carbon using Zn MOF and metal salts. Jaouen et al. identified a volcano trend in MNC catalysts with MNx (M = Mn, Co, Fe, Ni, and Cu), providing an in-depth understanding of the activity and selectivity of atomically MNx with different metals for CO2RR. MNC catalysts as promising candidates for CO2RR were studied as model catalysts. Co was observed at the top of the volcano based on electrochemical potential. The experimental-operando X-ray absorption near edge structural spectroscopy was used for accurate modelling of active sites keeping the changes in the oxidation states of metals with change in potential. CoNC had no change in oxidation state with changing potential, and M2 + N4-H2O was identified as the most active center by computational studies. This work provided a base for the design and fabrication of cobalt-based MNC catalysts for CO2RR to be used in the future [43][45]. The cobalt nitrogen functionalized materials are noteworthy catalysts for CO2RR due to their high-performance activities. Metalloporphyrin and its derivatives have also been promising materials for catalytic activities [12][14]. Therefore, in 2019, Zhou et al. employed DFT calculations to study CO2RR on Co-centered porphyrin and graphene with C, N and O as different coordinating atoms to further improve the activities. Through coordination engineering, the catalytic activities can be enhanced by the cobalt atom’s vacancy formation energy arising by substituting different coordinating atoms. Detailed electronic studies results showed that Co-O bonds lack π
-bonding compared to the Co-N and Co-C bonds in the Co-centered structure, therefore, had potential for high catalytic activities. Hence, coordination engineering can be employed as an effective strategy for the enhancement of CO2RR catalytic activities in cobalt nitrogen functionalized materials [105][147].
2.5. Cobalt-Based Complexes for CO
2
RR
Transition metal complexes offer an advantage for CO2RR due to fine-tuning the coordination sphere via altering the chelating surroundings vis-à-vis electronic and steric effects of the chelating agents. Such fine-tuning is not possible in solid-state transition metal catalysts. Metal complex-type catalysts are available in the literature, ranging from noble metals (Ir, Ru, Re, etc.) to none-noble coinage metals (Co, Ni, Fe Cu, etc.) [106][107][150,151]. These metals provide a two-electron reduction pathway to form -COOH, using organic reaction media. The porphyrin ring is an efficient ligand among other ligands because of its peculiar stability and high photo-electrochemical traits. A variety of cobalt complexes have been investigated for CO2RR with promising results, there is still a need to find and generate a low-valent intermediate with significantly lower potential.
2.6. Cobalt Porphyrin for CO
2
RR
In recent years, porphyrin-based metal complexes have been significantly explored for CO2 reduction [108][155]. This section keeps separately due to its broad research because porphyrin-ring offers high electron transfer activity, thus requiring relatively lower overpotential with high CO selectivity in the CO2 reduction reactions. Additionally, researchers have made significant progress in porphyrin-based electrocatalysts anchored on potential host materials in terms of catalyst stability. Immobilized cobalt porphyrins have been reported for high faraday efficiency towards CO with lower overpotential. First principle calculations have also played a pivotal part in explaining the electronic structures of the metal-porphyrin catalytic systems for CO2 reduction [109][156]. Shen et al. [68][70] immobilized cobalt protoporphyrins on pyrolytic graphite in aqueous media, and tested them for CO2 reduction. The authors underpinned the pH stability of cobalt porphyrin electrocatalyst, saying that optimization of pH plays a crucial role in triggering the CO2 reduction reaction. Their study claimed a >60% Faraday efficiency towards CO and >2.5% towards CH4 was reported at a potential of −0.6 V vs. RHE, under acidic conditions. First principle calculations are important to understand the reaction pathways in the cobalt porphyrin electrocatalytic systems. In this regard, Leung et al. [110][157] performed the pioneering work by applying quantum chemistry and ab initio calculations in investigating the electrocatalytic effect of cobalt porphyrin complexes in the reductive decomposition of CO2 in aqueous media. They used hybrid functionals alongside the dielectric continuum solcation methods, for determining the electron transfer mechanisms. They proposed a reductive mechanism that explained the CO2 reduction at ambient to higher pH levels, which starts with one-electron transfer to the cobalt porphyrin complex [Co-porphyrin]−, which then adds CO2 to itself forming adjunct an [Co-porphyrin.CO2]2−. Upon protonation an intermediate [Co-porphyrin.COOH]− is produced, which upon relieving the OH− moiety, produces the product CO. The two key intermediates in this whole reaction cycle are [Co-porphyrin.CO2]2 and [Co-porphyrin.COOH]−, due to the stronger interactions of CO2 and water. Miyamoto and Asahi [111][158] reported on the enhanced CO2 reduction to CO over cobalt porphyrin complexes in water based upon Koper’s mechanism. They combined their DFT calculations with the experimental results. Their work targeted the pH stability of their catalytic system, and reported that at pH = 3, a single proton shift from water occurs at −0.8 V vs. RHE. They said that water plays a vital role in the CO2 reduction to CO, as their calculated redox potential of proton transfer matched that of the experimental results.2.7. Cobalt-Based MOFs and COFs for CO
2
RR
The reticular chemistry of the MOFs and COFs enable the use of tunable control of the catalytic system to convert the carbon dioxide to value-added products [112][159]. However, this tunable property is prohibited by poor electrical conductivities. This can only be overcome by the suitable use of the metals such as cobalt, copper in the inorganic SBUs.Katherine A. Mirica reported the synthesis of conductive two-dimensional MOF made of metallophalocyanine (cobalt/nickel) ligands linked by copper nodes with high electrical conductivities for the conversion of CO2 to CO. It was observed that TOF values range from 1.15 and 0.63 s−1 for CoPc-Cu-NH and CoPc-Cu-O [113][160]. Christopher J. Chang reported COF synthesis containing cobalt porphyrin catalysts as building units and organic linkers bonded through imine linkage to make the COF for the aqueous electrochemical reduction of CO2 to CO. The COF materials were deposited on porous, conductive carbon fabric. Incorporating tubular molecular units of the porphyrins within the extended COF structure gave an advantage in electrocatalytic reduction with exceptionally high activity and selectivity. Thus we find that with the increasing length of the linker from COF-366 to COF-367, the activity is increased with the TON up to 290,000 and TOF of 9400 h−1 about 26-fold more than the normal cobalt complex with no degradation over 24 h [114][161]. Rong Xu immobilized cobalt oxide nanoparticles on MIL-101 for water oxidation with a TOF of 0.012 s−1 [115][162]. Jinhong et al. prepared cobalt immobilized on a covalent triazine-based framework (CTF) as an efficient cocatalyst to reduce CO2 under visible-light irradiation. The CTF helps in the CO2 adsorption while the pore structure helps in the accommodation of CO2 and electron mediator. It was observed that the production of CO increases 44-fold than the pristine CTF on the introduction of cobalt. The obtained CO from this catalyst was about 50 mol g−1h−1 [116][163]. Peidong Yang et al. prepared a cobalt porphyrin-based Aluminum MOF for the conversion of CO2 to CO with the TON up to 1400. In situ analysis showed that the majority of the redox-accessible Co(II) is reduced to Co(I) during catalysis [117][164]. Xinyong Li prepared a novel Z-scheme heterojunction of Co3O4@CoFe2O4 hierarchical hollow double-shelled nanoboxes derived from ZIF-67 to give CH4 and CO at a rate of 2.06 mol h−1 and 72.2 mol h−1, respectively [117][164]. Yaghi et al. [118][165] prepared a new anionic 3D metal–organic framework MOF-1992 containing Co phthalocyaninoctaol. It converts CO2 to CO with the TON up to 5800 and TOF 0.20 s−1 with a current density of −16.2 mA cm−2 at −0.52. The electroactive coverage of the catalyst was estimated with the aid of the CV measurement (Figure 310), and it was found to be ~25% of the total catalyst loading. Zhang et al. [119][166] studied a novel mixed-metallic MOF [Ag4Co2(PYZ)PDC4] which transformed into an Ag-doped CoO4 catalyst. The Ag/Co3O4 catalyst gives the highest selectivity for CO in 0.1 M KHCO3 electrolyte (CO2 saturation), up to ~45% Faradaic efficiency was reported. Compared to the Ag/Co3O4 electrode, the highest Faradaic efficiency for CO over the pure Co3O4 is 21.3% at 1.8 V (vs. SCE). Their findings show that the presence of Ag improves the efficiency of CO significantly and inhibits H2 production for 10 h at −1.8 V (vs. SCE). Pan et al. [38][40] used cobalt MOF as a precursor to synthesized electrocatalysts on carbon as model catalysts for CO2RR. The prepared Co electrocatalyst was compared with Fe MOF-based electrocatalysts. The MOFs-derived catalysts were more active than the bulk electrocatalysts for CO2 reduction over hydrogen production reaction.
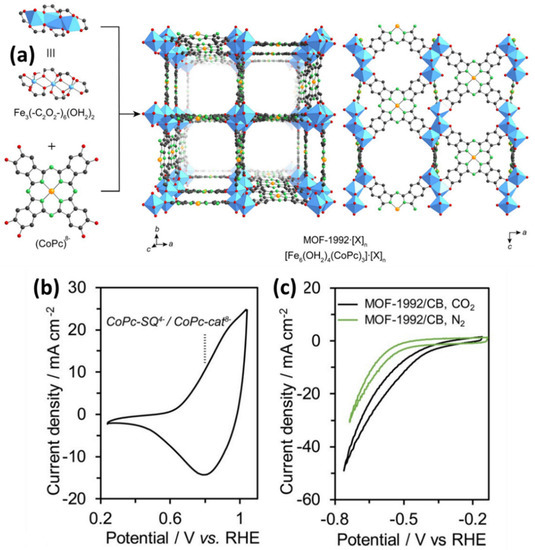

Figure 310. (a) Single-crystal X-ray structure of MOF-1992 based on the Fe-trimers and Co-phthalocyanine catechollinkers (CoPc). Atoms color: C-black; O-red; N-green; Co-orange; Fe-blue polyhedra. Hydrogen atoms and the chlorido ligands are omitted. The anionic charge of [Fe6(OH2)4(CoPc)3]6-, MOF-1992, was balanced by the presence of [X] n counterions (X represents Mg2+ or Fe3+). Electrochemical characterizations of the MOF-1992 (b) Cyclic voltammetry (CV) of the MOF-1992/CB (CB, carbon black). The vertical line display the potential of Co-Pc-semiquinolate (CoPc-SQ) 4-/CoPc-catecholate (CoPc-cat) 8-redox couple (c) CV of the MOF-1992/CB in a CO2-saturated (black, pH = 6.8) and N2-saturated (green, pH = 7.2) KHCO3 solution. Reproduced from [118][165], with permission from American Chemical Society, 2019.
3. Outlook
Despite several advancements, further improvements for recommendation are given below:
-
Mixed metal catalysts: The numbers of electrons involved in the ECO2RR are 2,8,12,14 for the formation of CO, methane, ethylene, and ethane, respectively. Bi and trimetallic catalysts are required to allow the charge transfer for the desire products especially CO2, towards higher hydrocarbons.
-
Complexes: The use of organometallic complexes is still needed to increase the conjugation of the materials to overcome the conductivity problem for CO2RR for products higher than the eight-electron system.
-
Nanostructured electrocatalysts: The use of nanostructured cobalt catalysts can be further improved to gain a high current and to provide a high density of active sites for CO2RR.
-
In situ/operando measurements: It is crucial to understand the catalyst’s active sites, reduction pathway, and the type of intermediates by operando techniques.
-
Simulation data: The CO2 electro reduction can be combined with simulation electrocatalysts to predict the product efficiency. A better reaction is needed to predict the catalyst development for efficient CO2RR.
-
Cobalt sulfide and selenide: The composites of cobalt sulfide and selenide can be explored for electrochemical CO2RR.
-
CO2 reduction under impurities: The post-combustion plant contains several impurities along with CO2. Thus CO2RR in the presence of impurities must be tested to revealed electrode activity and stability.
-
Mixed gases data: CO2 in the air is present as a mixture with other gases. The practical application of this technology is required to reduce the CO2 in mixed gases electrochemically.
-
Current density: Currently, cobalt-based materials used liquid electrolytes with bubbled CO2 for reduction. However, this gives low current density. The gas diffusion cells can be introduced to overcome this solubility issue by the liquid electrolytes and to achieve high current densities (>100 mA).
-
Use of solid electrolytes: A solid-state electrolyte configuration might overcome the challenge of CO2 solubility and low current density.
