Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Sahmin Lee.
Angiotensin receptor neprilysin inhibitor (ARNI) treatment reduces functional mitral regurgitation (MR) to a greater extent than angiotensin receptor blocker (ARB) treatment alone, but the mechanism is unclear.
- neprilysin
- angiotensin receptor antagonists
- mitral valve insufficiency
1. Introduction
After myocardial infarction (MI), tethering and fibrosis of mitral leaflets stimulate functional mitral regurgitation (MR), resulting in high morbidity of heart failure (HF) and cardiac mortality [1,2,3,4,5,6,7,8][1][2][3][4][5][6][7][8]. As secondary functional MR usually develops as a result of left ventricular (LV) dysfunction [1,6][1][6], medications for HF such as beta blockers, angiotensin-converting-enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) are the mainstay of medical therapy for functional MR [9,10][9][10]. However, the pharmacological treatment has not been found to be sufficient for reducing ischemic MR or reversing the adverse LV remodeling [5,11,12][5][11][12].
Post-MI changes in the mitral valve (MV) are associated not only with LV remodeling, but also with an excessive endothelial-to-mesenchymal transition (EndoMT) by transforming growth factor-β (TGF-β) overexpression [13,14][13][14]. Fibrotic remodeling and thickened leaflet of MV make leaflet area insufficient and cause inadequate adaptation, which finally results in coaptation failure of mitral leaflets and facilitates functional MR [15,16,17,18,19,20][15][16][17][18][19][20]. Therefore, inhibition of EndoMT by blocking TGF-β expression or its downstream signaling may offer a strategy to prevent inadequate adaptations and effectively mitigate functional MR.
Reducing functional MR is highly beneficial to the clinical outcomes of patients with MI or HF. A recent randomized trial found that reduction of functional MR by transcatheter MV repair resulted in a lower rate of hospitalization for HF and lower mortality in patients with HF and significant secondary MR [21]. Furthermore, we recently performed a double-blind, randomized clinical trial, which demonstrated that angiotensin receptor-neprilysin inhibitor (ARNI) treatment effectively reduced functional MR more than ARB alone in patients with LV dysfunction [22]. Although ARNI therapy has shown potential to promote reverse remodeling of LV in this clinical trial, little is known about the mechanism responsible for the beneficial action of neprilysin inhibition on functional MR.
ARNI is a combination drug of sacubitril, a neprilysin inhibitor, and valsartan, an ARB [23]. In the PARADIGM-HF trial [24], ARNI treatment was found to have substantial benefits in reducing all-cause mortality and hospitalization among HF patients with reduced ejection fraction when compared to enalapril, an ACE inhibitor. In addition to the effect as an ARB, the mechanism of beneficial action of neprilysin inhibition includes enhancement of endogenous natriuretic peptide, which facilitates sodium excretion and has vasodilating effects [25[25][26],26], and inhibition of cardiac fibrosis and hypertrophy [27]. Natriuretic peptides are hormones produced from heart or vascular endothelium in response to preload or afterload, and fluid retention through specific receptors [28].
2. Left Ventricular Remodeling after Myocardial Infarction
Two weeks after occluding the left circumflex coronary artery in SD rats, significant post-MI LV dilatation was confirmed (Figure 1A) along with wall motion abnormality and ischemic fibrosis in posterolateral wall of LV (Figure 1B,C). The schematic of the study protocol is illustrated in Figure 1D. There was no significance difference in LV dilation and systolic function between the three groups at 2 weeks (Figure 1E). Six weeks after MI and randomization, all of 31 experimental rats survived well and there was no significant difference in serial changes of body weight between the three groups; LCZ696 treatment, valsartan treatment, and MR control group (Figure 1F).
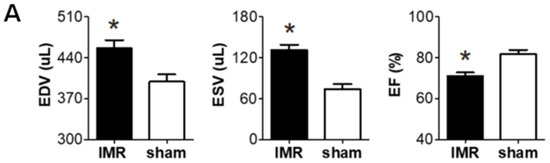
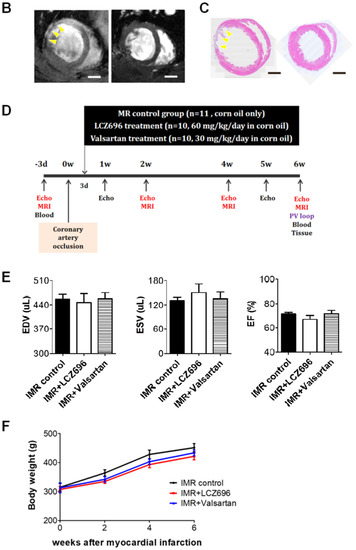


Figure 1. (A) Two weeks after left circumflex coronary artery occlusion was performed to induce myocardial infarction and ischemic mitral regurgitation (IMR), left ventricle size and function were evaluated by magnetic resonance imaging (MRI). (B,C) Representative images of cardiac MRI and immunohistochemical staining of hearts from the IMR model group (left) and sham control group (right). Arrows indicate infarcted area and myocardial fibrosis. Scale bar = 3 mm. (D) Schematic illustration of animal experiment protocol. Rats were randomly assigned to LCZ696 treatment (sacubitril/valsartan, 60 mg/kg/day in corn oil, n = 10), valsartan treatment (30 mg/kg/d in corn oil, n = 10), or corn oil only (MR control group; n = 11) group. (E) Comparison of parameters at 2 weeks after myocardial infarction among the three groups. (F) Comparison of serial changes in body weight among the three groups. EDV indicates end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction; MRI, magnetic resonance imaging; IMR, ischemic mitral regurgitation. * p < 0.05 for differences from the sham group.
3. Neprilysin Inhibitor Facilitates Left Ventricular Reverse Remodeling
LCZ696 significantly attenuated post-MI LV dilatation after 6 weeks when compared with the control group (LV EDV, 461.3 ± 13.8 µL versus 525.1 ± 23.6 µL; p < 0.05), which was assessed by means of cardiac MRI, while valsartan did not (LV EDV, 471.2 ± 8.9 µL; p > 0.05 to control) (Figure 2A,B). Echocardiography also showed significant reduction of LV end-diastolic dimension (EDD) in LCZ696 group (8.5 ± 0.2 mm in the LCZ696 group versus 9.1 ± 0.2 mm in the control group; p < 0.05) (Figure 2C,D). LCZ696 treatment decreased post-MI fibrosis in the LV myocardium (Figure 2E,F) as well as gross heart weight compared to the control group (1.4 ± 0.03 g versus 2.1 ± 0.17 g; p < 0.05), whereas valsartan did not (1.6 ± 0.10 g; p > 0.05) (Figure 2G).
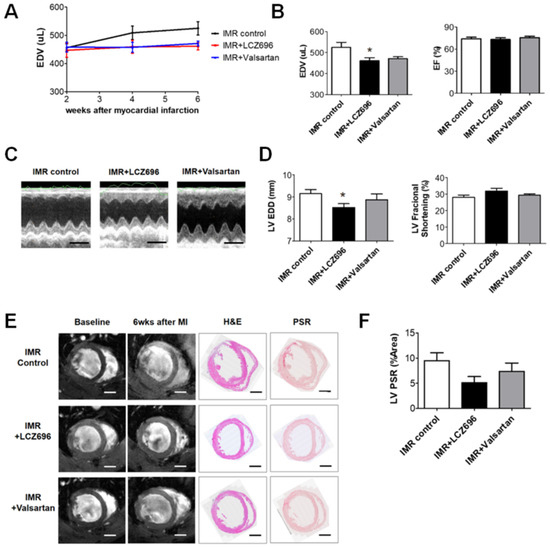
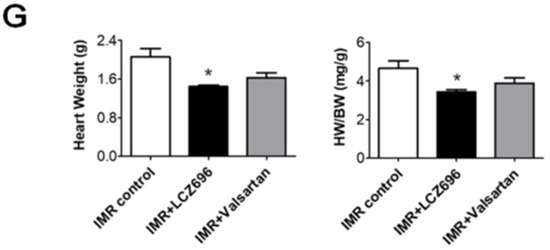


Figure 2. (A) Comparison of serial changes in end-diastolic volume (EDV) of the left ventricle until 6 weeks after myocardial infarction (MI) among the three groups. (B) Quantitative graphs of cardiac magnetic resonance imaging (MRI) measurements, which showed that EDV was significantly lower in the LCZ696 group than in the control group. There were no significant differences between the valsartan group and the control group. (C) Comparison of representative M-mode images acquired by echocardiogram at 6 weeks after MI from the three groups. Scale bar = 5 mm. (D) Quantitative graphs of echocardiographic measurements, which showed that end-diastolic dimensions were also significantly lower in the LCZ696 group than in the control group. (E) Representative images of cardiac MRI at baseline and 6 weeks after MI and immunohistochemistry at 6 weeks from the three groups. Scale bar = 3 mm. (F) A quantitative graph, which shows that the extent of myocardial fibrosis was smaller in the LCZ696 group than in the control group. LV PSR means overall fibrotic area of LV myocardium regardless of infarct size. (G) The LCZ696 group also had significantly lower heart weight than the control group, while valsartan did not. EDV indicates end-diastolic volume; IMR, ischemic mitral regurgitation; EF, ejection fraction; LV, left ventricle; EDD, end-diastolic dimension; wk, week; MI, myocardial infarction; H&E, Hematoxylin and eosin stain; PSR, picrosirius red stain; HW, heart weight; BW, body weight. * p < 0.05 for differences from the control group.
References
- Pierard, L.A.; Carabello, B.A. Ischaemic mitral regurgitation: Pathophysiology, outcomes and the conundrum of treatment. Eur. Heart J. 2010, 31, 2996–3005.
- Lamas, G.A.; Mitchell, G.F.; Flaker, G.C.; Smit, S.C., Jr.; Gersh, B.J.; Basta, L.; Moyé, L.; Braunwald, E.; Pfeffer, M.A. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and ventricular enlargement investigators. Circulation 1997, 96, 827–833.
- Bursi, F.; Enriquez-Sarano, M.; Nkomo, V.T.; Jacobsen, S.J.; Weston, S.A.; Meverden, R.A.; Roger, V.L. Heart failure and death after myocardial infarction in the community: The emerging role of mitral regurgitation. Circulation 2005, 111, 295–301.
- Rossi, A.; Dini, F.L.; Faggiano, P.; Cicoira, M.; Frattini, S.; Simioniuc, A.; Gullace, M.; Ghio, S.; Enriquez-Sarano, M.; Temporelli, P.L. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011, 97, 1675–1680.
- Levine, R.A.; Schwammenthal, E. Ischemic mitral regurgitation on the threshold of a solution: From paradoxes to unifying concepts. Circulation 2005, 112, 745–758.
- Asgar, A.W.; Mack, M.J.; Stone, G.W. Secondary mitral regurgitation in heart failure: Pathophysiology, prognosis, and therapeutic considerations. J. Am. Coll. Cardiol. 2015, 65, 1231–1248.
- Agricola, E.; Ielasi, A.; Oppizzi, M.; Faggiano, P.; Ferri, L.; Calabrese, A.; Vizzardi, E.; Alfieri, O.; Margonato, A. Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur. J. Heart Fail. 2009, 11, 581–587.
- Grigioni, F.; Detaint, D.; Avierinos, J.F.; Scott, C.; Tajik, J.; Enriquez-Sarano, M. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 260–267.
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, e57–e185.
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Munoz, D.R.; et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791.
- Doughty, R.N.; Whalley, G.A.; Walsh, H.A.; Gamble, G.D.; Lopez-Sendon, J.; Sharpe, N.; Investigators, C.E.S. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: The capricorn echo substudy. Circulation 2004, 109, 201–206.
- Solomon, S.D.; Skali, H.; Anavekar, N.S.; Bourgoun, M.; Barvik, S.; Ghali, J.K.; Warnica, J.W.; Khrakovskaya, M.; Arnold, J.M.O.; Schwartz, Y.; et al. Changes in ventricular size and function in patients treated with valsartan, captopril, or both after myocardial infarction. Circulation 2005, 111, 3411–3419.
- Levine, R.A.; Hagége, A.A.; Judge, D.P.; Padala, M.; Dal-Bianco, J.P.; Aikawa, E.; Beaudoin, J.; Bischoff, J.; Bouatia-Naji, N.; Bruneval, P.; et al. Mitral valve disease—Morphology and mechanisms. Nat. Rev. Cardiol. 2015, 12, 689–710.
- Li, Y.; Lui, K.O.; Zhou, B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat. Rev. Cardiol. 2018, 15, 445–456.
- Debonnaire, P.; Al Amri, I.; Leong, D.P.; Joyce, E.; Katsanos, S.; Kamperidis, V.; Schalij, M.J.; Bax, J.J.; Marsan, N.A.; Delgado, V. Leaflet remodelling in functional mitral valve regurgitation: Characteristics, determinants, and relation to regurgitation severity. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 290–299.
- Connell, P.S.; Azimuddin, A.F.; Kim, S.E.; Ramirez, F.; Jackson, M.S.; Little, S.H.; Grande-Allen, K.J. Regurgitation hemodynamics alone cause mitral valve remodeling characteristic of clinical disease states in vitro. Ann. Biomed. Eng. 2016, 44, 954–967.
- Beaudoin, J.; Dal-Bianco, J.P.; Aikawa, E.; Bischoff, J.; Guerrero, J.L.; Sullivan, S.; Bartko, P.E.; Handschumacher, M.D.; Kim, D.H.; Wylie-Sears, J.; et al. Mitral leaflet changes following myocardial infarction: Clinical evidence for maladaptive valvular remodeling. Circ. Cardiovasc. Imaging 2017, 10, e006512.
- Chaput, M.; Handschumacher, M.D.; Tournoux, F.; Hua, L.; Guerrero, J.L.; Vlahakes, G.J.; Levine, R.A. Mitral leaflet adaptation to ventricular remodeling: Occurrence and adequacy in patients with functional mitral regurgitation. Circulation 2008, 118, 845–852.
- Chaput, M.; Handschumacher, M.D.; Guerrero, J.L.; Holmvang, G.; Dal-Bianco, J.P.; Sullivan, S.; Vlahakes, G.J.; Hung, J.; Levine, R.A. Mitral leaflet adaptation to ventricular remodeling: Prospective changes in a model of ischemic mitral regurgitation. Circulation 2009, 120, S99–S103.
- Kunzelman, K.S.; Quick, D.W.; Cochran, R.P. Altered collagen concentration in mitral valve leaflets: Biochemical and finite element analysis. Ann. Thorac. Surg. 1998, 66, S198–S205.
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter mitral-valve repair in patients with heart failure. N. Engl. J. Med. 2018, 379, 2307–2318.
- Kang, D.H.; Park, S.J.; Shin, S.H.; Hong, G.R.; Lee, S.; Kim, M.S.; Yun, S.C.; Song, M.J.; Park, S.W.; King, J.J. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation 2019, 139, 1354–1365.
- Gu, J.; Noe, A.; Chandra, P.; Al-Fayoumi, S.; Ligueros-Saylan, M.; Sarangapani, R.; Maahs, S.; Ksander, G.; Gigel, D.F.; Jeng, Y.F.; et al. Pharmacokinetics and pharmacodynamics of lcz696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (arni). J. Clin. Pharmacol. 2010, 50, 401–414.
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.R.; Shi, C.V.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004.
- Hubers, S.A.; Brown, N.J. Combined angiotensin receptor antagonism and neprilysin inhibition. Circulation 2016, 133, 1115–1124.
- Sacks, C.A.; Jarcho, J.A.; Curfman, G.D. Paradigm shifts in heart-failure therapy—A timeline. N. Engl. J. Med. 2014, 371, 989–991.
- Von Lueder, T.G.; Wang, B.H.; Kompa, A.R.; Huang, L.; Webb, R.; Jordaan, P.; Ater, D.; Krum, H. Angiotensin receptor neprilysin inhibitor lcz696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ. Heart Fail. 2015, 8, 71–78.
- Kato, J. Natriuretic peptides and neprilysin inhibition in hypertension and hypertensive organ damage. Peptides 2020, 132, 170352.
More
