Nucleotide-binding oligomerization domain 2 (NOD2) is a cytoplasmic receptor that recognizes invading molecules and danger signals inside the cells.
- NOD2
- NLRs
- ER stress
- autophagy
- innate immunity 1. Introduction
1. Introduction
2. NLR Family and Structure
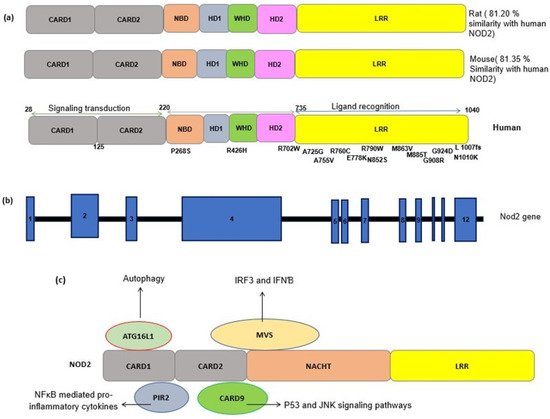
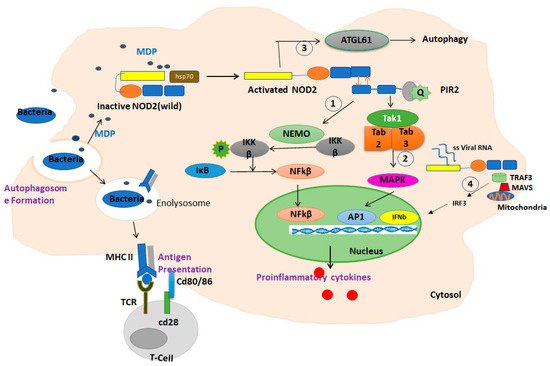
3. NOD2: Cellular and Molecular Mechanisms
4. NOD2 Genetics and Polymorphism
The human gene encoding for the NOD2 receptor is CARD15, located on chromosome 16q12.1. The NOD2 protein has 104 amino acids with a molecular weight of 110 kDa, which is a multifunctional receptor. As NOD2 has many important roles, mutations in its gene may have serious consequences in vital cellular functions and immunity. NOD2 is a repository of genetic variants, most of which are associated with pathological conditions. Many previous studies have reported the association of NOD2 polymorphisms with inflammatory diseases (Table 1) [23][22]. As LRR is the ligand binding domain of the NOD2 receptor, mutations in this region may affect either responses to MDP or the downstream pathways [69][23]. The nonsense mutations in this region also may abolish the conformational changes needed for MDP binding and receptor activation and thus may lead to receptor loss-of-function.Number |
|---|
SNPs |
|---|
Mutation |
|---|
Location |
|---|
Population |
|---|
Result |
|---|
Infection |
|---|
(Disease) |
|---|
Method |
|---|
Ref |
|---|
1 |
P268S |
CCC > TCC |
NBD |
domain |
African Americans |
Minor allele T is associated with a decreased risk of TB (Protective) |
Tuberculosis |
Sequencing of the coding regions of |
the NOD2 gene |
[ | ] |
[ | ] |
R702W |
CGG > TGG |
[ |
] |
4 |
HD2 |
Exon 4 |
Minor allele T is associated with a decreased risk of TB(Protective) |
A725G |
GCT > GGT |
HD2 |
Exon 4 |
the minor allele G increased the risk of TB |
2 |
R702W |
CGG > TGG |
South African |
No association |
Inflammatory bowel disease (CD & UC) |
PCR of the Exons 4, 8 and 11- HEX-SSCP &RFLP | |||
[ | ] |
[ | ||
] |
A725G |
GCT > GGT |
Increased risk of TB |
G908R |
Rs2066845 |
No association |
1007fs(insC3020) |
L1007P |
rs5743293 |
No association |
3 |
rs3135499 |
Promoter |
Han Chinese from Jiangsu Province |
T genotype protective |
Tuberculosis |
TaqMan-based |
allelic discrimination system |
[ |
] |
[ |
] |
rs7194886 |
Promoter |
Increased risk for T allele carriers |
rs8057341 |
Promoter |
rs9302752 |
Promoter |
T genotype protective |
4 |
insC3020 |
rs5743293 |
Sardinian population. |
Significant Association |
(Increased the susceptibility) |
CD & Mycobacterium avium subsp. paratuberculosis |
PCR & sequencing |
[ |
] |
[ |
] |
R702W |
Rs2066844 |
G908R |
Rs2066845 |
5 |
insC3020 |
1007fs |
northern Indian states |
No mutation was observed |
in the patients and controls |
TB and leprosy |
PCR-RFLP confirmed by gene sequencing |
[ |
] |
[ |
] |
R702W |
Rs2066844 |
G908R |
rs2066845 |
6 |
R702W |
South African |
No association |
Tuberculosis |
Tag Man platform genotyping |
[ |
] |
[ |
] |
G908R |
insC3020 |
7 |
P268S |
C > T |
rs2066842 |
Exon 4 |
Caucasian patients |
No association |
Sarcoidosis |
Tag Man platform genotyping |
[ |
] |
[ |
] |
R587R |
T > G |
rs1861759 |
Exon 4 |
R702W |
C > T |
rs2066844 |
Exon 4 |
G908R |
G > C |
rs2066845 |
Exon 8 |
insC3020 |
rs2066847 |
Exon 11 |
8 |
P268S |
Turkish population |
Association with CD |
Crohn’s Disease and Ulcerative Colitis |
PCR-RFLP |
[ |
] |
[ |
] |
M863V |
No mutant was found |
9 |
R702W |
rs2066844 |
CGG > TGG |
Meta analysis |
C allele is a risk factor |
sarcoidosis |
Meta-analysis |
[ |
] |
[ |
] |
G908R |
rs2066845 |
no associated |
insC3020 |
rs2066847 |
no associated |
R587R |
rs1861759 |
no associated |
10 |
C-159 T |
rs2569190 |
Meta analysis |
GG is common in TB |
Tuberculosis |
Meta-analysis |
[ |
] |
[ |
] |
A-1145G |
rs2569191 |
T allele is a risk factor in TB |
IV |
rs1861759 |
TG genotype is higher in TB |
rs7194886 |
T allele is a risk factor of TB |
R702W |
rs2066844 |
CC genotype is a risk factor for TB |
P 507 T/S |
rs2066842 |
C > A/T |
CC genotype is a risk factor for TB |
11 |
-159C > T |
-159C > T |
promoter of CD14 |
Chinese |
Higher risk |
increased promoter activity/increased sNOD2 |
spinal TB |
Seq. |
[ |
] |
[ |
] |
12 |
G-1619A |
rs2915863 |
promoter of CD14 |
Han Chinese |
Increased susceptibly/ |
increased sNOD2 |
tuberculosis |
PCR and seq |
[ |
] |
[ |
] |
T-1359G |
rs3138078 |
A-1145G |
rs2569191 |
C-159T |
rs2569190 |
13 |
C(-159)T |
promoter of CD14 |
Han Chinese |
T allele is a RF |
tuberculosis |
PCR-DNA sequencing |
[ |
] |
[ |
] |
G(-1145)A |
G allele is a RF |
14 |
C(-159)T |
promoter of CD14 |
increased level of serum soluble CD14 |
tuberculosis |
[ |
] |
[ |
] |
15 |
C(-159)T |
promoter of CD14 |
Mexico |
increased Tb susceptibility/ increased level of serum soluble CD14 |
PCR-RFLP |
[ |
] |
[ |
] |
16 |
C(-159)T |
Promoter |
Meta analysis |
increased risk of TB |
Meta-analysis |
[ |
] |
[ |
] |
17 |
R426H |
rs562225614 |
G > A |
Exon 4 |
Case report |
Early Onset Inflammatory Bowel Phenotype |
IBD-Increased expression of inflammatory cytokines |
Sequencing |
[ |
] |
[ |
] |
18 |
N1010K |
3030A > C |
LRR domain |
Exon 12 |
CD |
Sequencing |
[ |
] |
[ |
] |
References
- Carrillo, J.L.M.; García, F.P.C.; Coronado, O.G.; García, M.A.M.; Cordero, J.F.C. Physiology and Pathology of Innate Immune Response against Pathogens; IntechOpen: London, UK, 2017.
- Negroni, A.; Pierdomenico, M.; Cucchiara, S.; Stronati, L. NOD2 and inflammation: Current insights. J. Inflamm. Res. 2018, 11, 49.
- Proell, M.; Riedl, S.J.; Fritz, J.H.; Rojas, A.M.; Schwarzenbacher, R. The Nod-like receptor (NLR) family: A tale of similarities and differences. PLoS ONE 2008, 3, e2119.
- Dolasia, K.; Bisht, M.K.; Pradhan, G.; Udgata, A.; Mukhopadhyay, S. TLRs/NLRs: Shaping the landscape of host immunity. Int. Rev. Immunol. 2018, 37, 3–19.
- Ye, Z.; Ting, J.P. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr. Opin. Immunol. 2008, 20, 3–9.
- King, A.E.; Horne, A.W.; Hombach-Klonisch, S.; Mason, J.; Critchley, H.O. Differential expression and regulation of nuclear oligomerization domain proteins NOD1 and NOD2 in human endometrium: A potential role in innate immune protection and menstruation. Mol. Hum. Reprod. 2009, 15, 311–319.
- Strober, W.; Murray, P.J.; Kitani, A.; Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 2006, 6, 9–20.
- Roth, S.A.; Simanski, M.; Rademacher, F.; Schroder, L.; Harder, J. The pattern recognition receptor NOD2 mediates Staphylococcus aureus-induced IL-17C expression in keratinocytes. J. Investig. Dermatol. 2014, 134, 374–380.
- Trindade, B.C.; Chen, G.Y. NOD1 and NOD2 in inflammatory and infectious diseases. Immunol. Rev. 2020, 297, 139–161.
- Motta, V.; Soares, F.; Sun, T.; Philpott, D.J. NOD-like receptors: Versatile cytosolic sentinels. Physiol. Rev. 2015, 95, 149–178.
- Kersse, K.; Bertrand, M.J.; Lamkanfi, M.; Vandenabeele, P. NOD-like receptors and the innate immune system: Coping with danger, damage and death. Cytokine Growth Factor Rev. 2011, 22, 257–276.
- Franchi, L.; Park, J.H.; Shaw, M.H.; Marina-Garcia, N.; Chen, G.; Kim, Y.G.; Núñez, G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell. Microbiol. 2008, 10, 1–8.
- Strober, W.; Watanabe, T. NOD2, an intracellular innate immune sensor involved in host defense and Crohn’s disease. Mucosal Immunol. 2011, 4, 484–495.
- Morgulis, A.; Coulouris, G.; Raytselis, Y.; Madden, T.L.; Agarwala, R.; Schäffer, A.A. Database indexing for production MegaBLAST searches. Bioinformatics 2008, 24, 1757–1764.
- Jacob, F.; Vernaldi, S.; Maekawa, T. Evolution and conservation of plant NLR functions. Front. Immunol. 2013, 4, 297.
- Caruso, R.; Warner, N.; Inohara, N.; Nunez, G. NOD1 and NOD2: Signaling, host defense, and inflammatory disease. Immunity 2014, 41, 898–908.
- Shanahan, M.T.; Carroll, I.M.; Grossniklaus, E.; White, A.; von Furstenberg, R.J.; Barner, R.; Fodor, A.A.; Henning, S.J.; Sartor, R.B.; Gulati, A.S. Mouse Paneth cell antimicrobial function is independent of Nod2. Gut 2014, 63, 903–910.
- Homer, C.R.; Richmond, A.L.; Rebert, N.A.; Achkar, J.P.; McDonald, C. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn’s disease pathogenesis. Gastroenterology 2010, 139, 1630–1641.
- Brooks, M.N.; Rajaram, M.V.; Azad, A.K.; Amer, A.O.; Valdivia-Arenas, M.A.; Park, J.H.; Nunez, G.; Schlesinger, L.S. NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell. Microbiol. 2011, 13, 402–418.
- Dominguez-Martinez, D.A.; Nunez-Avellaneda, D.; Castanon-Sanchez, C.A.; Salazar, M.I. NOD2: Activation during bacterial and viral infections, polymorphisms and potential as therapeutic target. Rev. Investig. Clin. 2018, 70, 18–28.
- Negroni, A.; Colantoni, E.; Vitali, R.; Palone, F.; Pierdomenico, M.; Costanzo, M.; Cesi, V.; Cucchiara, S.; Stronati, L. NOD2 induces autophagy to control AIEC bacteria infectiveness in intestinal epithelial cells. Inflamm. Res. 2016, 65, 803–813.
- Beynon, V.; Cotofana, S.; Brand, S.; Lohse, P.; Mair, A.; Wagner, S.; Mussack, T.; Ochsenkühn, T.; Folwaczny, M.; Folwaczny, C. NOD2/CARD15 genotype influences MDP-induced cytokine release and basal IL-12p40 levels in primary isolated peripheral blood monocytes. Inflamm. Bowel Dis. 2008, 14, 1033–1040.
- Mukherjee, T.; Hovingh, E.S.; Foerster, E.G.; Abdel-Nour, M.; Philpott, D.J.; Girardin, S.E. NOD1 and NOD2 in inflammation, immunity and disease. Arch. Biochem. Biophys. 2019, 670, 69–81.
- Austin, C.M.; Ma, X.; Graviss, E.A. Common nonsynonymous polymorphisms in the NOD2 gene are associated with resistance or susceptibility to tuberculosis disease in African Americans. J. Infect. Dis. 2008, 197, 1713–1716.
- Zaahl, M.; Winter, T.; Warnich, L.; Kotze, M. Analysis of the three common mutations in the CARD15 gene (R702W, G908R and 1007fs) in South African colored patients with inflammatory bowel disease. Mol. Cell. Probes. 2005, 19, 278–281.
- Pan, H.; Dai, Y.; Tang, S.; Wang, J. Polymorphisms of NOD2 and the risk of tuberculosis: A validation study in the Chinese population. Int. J. Immunogenet. 2012, 39, 233–240.
- Sechi, L.A.; Gazouli, M.; Ikonomopoulos, J.; Lukas, J.C.; Scanu, A.M.; Ahmed, N.; Fadda, G.; Zanetti, S. Mycobacterium avium subsp. paratuberculosis, genetic susceptibility to Crohn’s disease, and Sardinians: The way ahead. J. Clin. Microbiol. 2005, 43, 5275–5277.
- Singh, V.; Gaur, R.; Mittal, M.; Biswas, S.; Das, R.; Girdhar, B.; Bajaj, B.; Katoch, V.; Kumar, A.; Mohanty, K. Absence of nucleotide-binding oligomerization domain-containing protein 2 variants in patients with leprosy and tuberculosis. Int. J. Immunogenet. 2012, 39, 353–356.
- Möller, M.; Nebel, A.; Kwiatkowski, R.; van Helden, P.D.; Hoal, E.G.; Schreiber, S. Host susceptibility to tuberculosis: CARD15 polymorphisms in a South African population. Mol. Cell. Probes 2007, 21, 148–151.
- Sato, H.; Williams, H.; Spagnolo, P.; Abdallah, A.; Ahmad, T.; Orchard, T.; Copley, S.; Desai, S.; Wells, A.; Du Bois, R. CARD15/NOD2 polymorphisms are associated with severe pulmonary sarcoidosis. Eur. Respir. J. 2010, 35, 324–330.
- Diler, S.B.; Polat, F.; Yaraş, S. The P268S and M863V Polymorphisms of the NOD2/CARD15 gene in Crohn’s disease and ulcerative colitis. Cytol. Genet. 2019, 53, 424–429.
- Chen, X.; Zhou, Z.; Zhang, Y.; Cheng, X.; Guo, X.; Yang, X. NOD2/CARD15 gene polymorphisms and sarcoidosis susceptibility: Review and meta-analysis. Sarcoidosis Vasc. Diffus. Lung Dis. 2018, 35, 115.
- Cubillos-Angulo, J.M.; Fernandes, C.D.; Araújo, D.N.; Carmo, C.A.; Arriaga, M.B.; Andrade, B.B. The influence of single nucleotide polymorphisms of NOD2 or CD14 on susceptibility to tuberculosis: A systematic review. Syst. Rev. 2021, 10, 174.
- Zheng, M.; Shi, S.; Wei, W.; Zheng, Q.; Wang, Y.; Ying, X.; Lu, D. Correlation between MBL2/CD14/TNF-α gene polymorphisms and susceptibility to spinal tuberculosis in Chinese population. Biosci. Rep. 2018, 38.
- Xue, Y.; Zhao, Z.; Chen, F.; Zhang, L.; Li, G.; Ma, K.; Bai, X.; Zuo, Y. Polymorphisms in the promoter of the CD14 gene and their associations with susceptibility to pulmonary tuberculosis. Tissue Antigens 2012, 80, 437–443.
- Zhao, M.; Xue, Y.; Zhao, Z.; Li, F.; Fan, D.; Wei, L.; Sun, X.; Zhang, X.; Wang, X.; Zhang, Y. Association of CD14 G(-1145)A and C(-159)T polymorphisms with reduced risk for tuberculosis in a Chinese Han population. Genet. Mol. Res. 2012, 11, 3425–3431.
- Alavi-Naini, R.; Salimi, S.; Sharifi-Mood, B.; Davoodikia, A.; Moody, B.; Naghavi, A. Association between the CD14 gene C-159T polymorphism and serum soluble CD14 with pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2012, 16, 1383–1387.
- Rosas-Taraco, A.G.; Revol, A.; Salinas-Carmona, M.C.; Rendon, A.; Caballero-Olin, G.; Arce-Mendoza, A.Y. CD14 C(-159)T polymorphism is a risk factor for development of pulmonary tuberculosis. J. Infect. Dis. 2007, 196, 1698–1706.
- Miao, R.; Ge, H.; Xu, L.; Xu, F. CD14–159C/T polymorphism contributes to the susceptibility to tuberculosis: Evidence from pooled 1700 cases and 1816 controls. Mol. Biol. Rep. 2014, 41, 3481–3486.
- Girardelli, M.; Loganes, C.; Pin, A.; Stacul, E.; Decleva, E.; Vozzi, D.; Baj, G.; De Giacomo, C.; Tommasini, A.; Bianco, A.M. Novel NOD2 mutation in early-onset inflammatory bowel phenotype. Inflamm. Bowel Dis. 2018, 24, 1204–1212.
- Frade-Proud’Hon-Clerc, S.; Smol, T.; Frenois, F.; Sand, O.; Vaillant, E.; Dhennin, V.; Bonnefond, A.; Froguel, P.; Fumery, M.; Guillon-Dellac, N. A novel rare missense variation of the NOD2 gene: Evidences of implication in Crohn’s disease. Int. J. Mol. Sci. 2019, 20, 835.
