The kidneys are vital organs performing several essential functions. Their primary function is the filtration of blood and the removal of metabolic waste products as well as fluid homeostasis. Renal filtration is the main pathway for drug removal, highlighting the importance of this organ to the growing field of nanomedicine. The kidneys (i) have a key role in the transport and clearance of nanoparticles (NPs), (ii) are exposed to potential NPs’ toxicity, and (iii) are the targets of diseases that nanomedicine can study, detect, and treat.
- nanomedicine
- nanotechnology
- nanoparticle
- kidney
- renal imaging
- tissue engineering
- drug delivery
1. Introduction
One of the main factors influencing the pharmacokinetics of drugs and therapeutic materials is the clearance rate from the body. After their absorption, nanoparticles enter the systemic circulation, diffuse and interact with the body, and are eventually cleared by the reticuloendothelial system (RES) or the kidney. In this Hereviewin, we will focus on the kidney’s influence, as renal excretion is one of the most critical factors involved in the excretion of nanomedicines. For more information regarding the optimization of nanodrug pharmacokinetics, we recommend the review by Moss and Siccardi [1].
The kidneys receive 1 to 1.2 L of blood per minute, that is 20–25% of the cardiac output. Each kidney contains an average of one million nephrons, the basic functional unit of the kidney. Each nephron consists of the renal corpuscle, or glomerulus, the proximal, and distal tubules. Blood is transported via the renal vasculature to the glomeruli, where the primary filtrate is passed through fenestrated capillaries into the Bowman’s capsule, which encloses the glomerulus and collects the filtrate. The filtrate is then transported to the proximal and distal renal tubules where nutrients, water, and ions are reabsorbed and waste products secreted.
From the lumen of the capillary to the inside of the Bowman’s capsule, the glomerulus contains four structures that act as filters: the glycocalyx, the endothelium, the glomerular basement membrane (GBM), and the podocytes. The glycocalyx is a negatively charged layer of glycoproteins and glycolipids that protects the underlying endothelial cells and prevents large protein leakage. The endothelial layer is a fenestrated endothelium with large pores of 70–90 nm in diameter. The endothelial cells repose on the negatively charged GBM with pores of 2–8 nm. Its role is to prevent the filtration of large proteins and cellular components from the blood. The last filtration barrier is composed of the podocytes that sit on the urine side of the GBM. Organized as a monolayer, their interdigitating foot processes form the filtration slit sized 4 to 11 nm. Overall, the four layers create a charge- and size-selective filtration barrier with a glomerular functional barrier ’s (GFB) pore size of 4.5–5 nm [2].
Size, shape, and charge of nanoparticles must be considered when designing nanomedicine, as they will decide between renal clearance and accumulation. For example, a positively charged nanomedicine may cross more efficiently the filtration through a negatively charged GBM and podocytes than neutral or negatively charged compounds. An overview of the potential interaction of NP with the kidney is shown in Figure 1 .
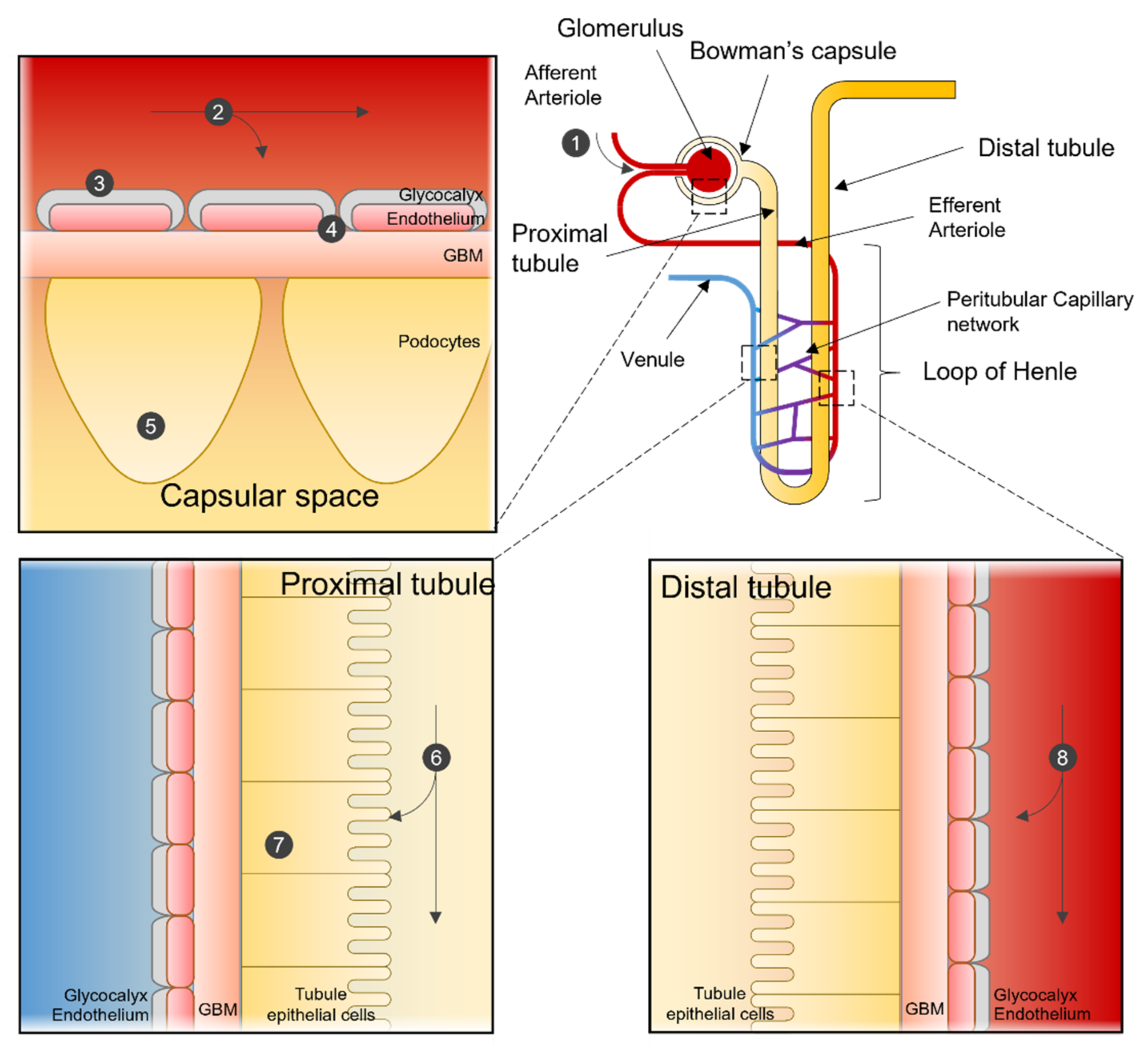 Figure 1. Nanoparticle interactions in the Nephron. Sites of interaction and potential uptake of NPs that are subject to extraction or used to target the kidney. NPs enter the kidney via the renal artery and are transported to the afferent arteriole (1). Depending on particle characteristics, NPs remain in the blood stream or are subjected to renal filtration from the blood in the glomerular capillaries (2). NPs can be designed so that renal structures, such as the glycocalyx (3), endothelial cells, or the glomerular basement membrane (4), can function in selecting NPs for filtration. After filtration, NPs can interact with podocytes in the Bowman’s lumen (5). NPs in the filtrate are then transported to the proximal tubule, where they interact with proximal epithelial cells (6) and are potentially reabsorbed. Pro-drug NPs can be activated in the lysosome of proximal tubular cells (7). NPs not selected for renal filtration can interact with the renal tubular comparted after being transported from the efferent arteriole to the peritubular network (8).
Figure 1. Nanoparticle interactions in the Nephron. Sites of interaction and potential uptake of NPs that are subject to extraction or used to target the kidney. NPs enter the kidney via the renal artery and are transported to the afferent arteriole (1). Depending on particle characteristics, NPs remain in the blood stream or are subjected to renal filtration from the blood in the glomerular capillaries (2). NPs can be designed so that renal structures, such as the glycocalyx (3), endothelial cells, or the glomerular basement membrane (4), can function in selecting NPs for filtration. After filtration, NPs can interact with podocytes in the Bowman’s lumen (5). NPs in the filtrate are then transported to the proximal tubule, where they interact with proximal epithelial cells (6) and are potentially reabsorbed. Pro-drug NPs can be activated in the lysosome of proximal tubular cells (7). NPs not selected for renal filtration can interact with the renal tubular comparted after being transported from the efferent arteriole to the peritubular network (8).
2. Nanotechnology for Renal Therapy
2.1. Drug Delivery
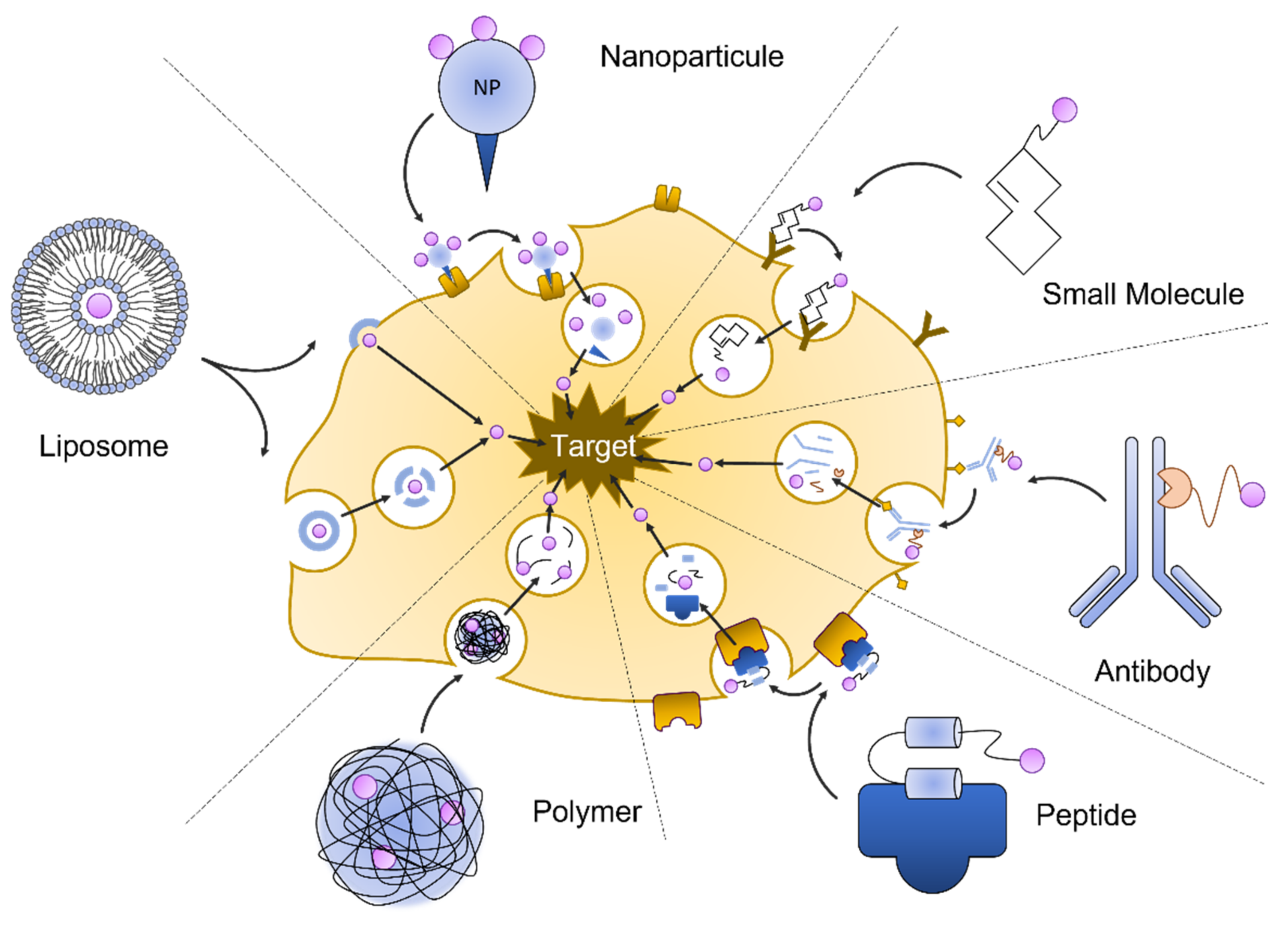
2.1.1. Liposomes
2.1.2. Antibodies
Biological enzymes, immunoglobulins, and peptide hormones are effective in targeting the kidney. Intra kidney delivery can be directed via immunoglobulin specificity or the uptake selectivity of peptides in different compartments. Complement activation is part of the immune response and provides an initial response against infections. The dysregulation of the activated complement system is a component in many renal diseases, such as glomerulopathies, ischemia/reperfusion injury, and thrombotic microangiopathies. Macrophage antigen 1 (Mac-1) is a receptor mediating complement-activated inflammation and is expressed predominantly on macrophages infiltrating sites of tissue damage and inflammation. Mac-1 consists of integrin aM (CD11b) and integrin 2 (CD18). Shirai et al., conjugated a CD11b antibody with silica nanoparticles. After injection in mice with experimental renal obstruction, he found that the antibody constructs bound with high affinity to the macrophages located to the area of tubular damage and interstitial inflammation [5]. Renal endothelial cells are an important part of the renal filtration apparatus. They are the target of complement activated injury during transplant-associated ischemia, leading to a delayed posttransplant function. Durigutto et al., generated an antibody specific for the complement factor C5 that was coupled with a cyclic RGD peptide [6]. The peptide RGD-4C has been found in a phage display assay to confer the binding to integrins expressed on endothelial cells. They found that in a rat model for I/R injury, the injected anti C5 antibody RGD conjugate was localized to the endothelial cells, where it prevented complement-activated injury. The antibody conjugate is proposed to be used in transplant medicine, where it could be used to pre-treat transplant organs from I/R associated injury.2.1.3. Protein/Peptide-Based Carrier
During glomerular filtration, low molecular weight (LMW) proteins are excreted with the primary urine and reabsorbed by proximal tubular cells. Reabsorption by the proximal tubular cells is a receptor-mediated endocytosis. After endocytosis, proteins are transported to the lysosome and enzymatically degraded into smaller peptides. Studies using macromolecules, such as chitosan [7] and glucosamine [8], or folate [9] as carriers, have shown that these molecule carriers accumulate and release drugs specifically in the kidney. Chitosan is a polysaccharide isolated from crustaceans and has been used in several drug delivery approaches because of its great biocompatibility and degradability. It promotes transmembrane transport across epithelial barriers and has a high affinity to the renal proximal tubular cells. Prednisolone is a glucocorticoid drug with several applications in renal medicine. It is used to preserve renal function and control the inflammatory response in renal diseases, such as lupus nephritis and glomerulonephritis, and is used to reduce chronic allograft rejection. He et al., conjugated an LMW chitosan with prednisolone and tested this compound in rat models for a minimal change disease. They found that the chitosan-prednisolone conjugate predominantly accumulated in the kidney. The conjugate effectively reduced proteinuria in animals with nephritic diseases, and it helped normalize blood urea nitrogen (BUN), albumin, and serum creatinine [10].2.1.4. Small Molecule Pro-Drugs
While methods of targeted delivery aim to deposit a bioactive molecule to a specific cell or tissue, pro-drugs are medications that are designed to be activated only after reaching their target [11]. This can reduce adverse side effects and increase the effectiveness of a drug while minimizing the total drug amount per body weight. Pro-drugs are widely used in cancer treatment, but pro-drugs have also been recently developed to deliver active compounds to the kidney. The quinolone antibiotic ciprofloxacin is commonly used to treat urinary tract infections and is metabolized in the liver. By conjugating ciprofloxacin to a synthetic lysin-rich peptide (KKEEE)3K, Wischnjow et al., were able to redirect the ciprofloxacin from the liver to the kidney [12]. They found that the radiolabeled pro-drug accumulated entirely in the kidneys can thereby deliver the antibiotic to the bladder with high efficiency.2.1.5. Nanoparticles
In renal targeting with nanoparticles, particle size can be used to generally determine the target compartment of the kidney. Particles small enough to pass the glomerular filtration (5–7 nm) barrier can be used to target the tubular area, where they are reabsorbed. Particles sized 30–150 nm cannot extravasate into the primary urine and remain in circulation [13]. Maximum glomerular accumulation has been described with a particle size of 80 nm. There are exceptions. For example, ultra-small particles (~2 nm) are too small to pass the glycocalyx and be removed via the liver. Mesoscale nanoparticles (MNP) (~400 nm) were found in proximal tubular cells, seemingly transported there by endocytosis because at that size, they are larger than the endothelial fenestration.2.1.6. Hydropolymeric Carriers
2.2. Tissue Engineering
2.2.1. Renal Replacement Technology
2.2.2. Kidney Regeneration
Rental transplantation is the treatment of choice for patients with end-stage kidney disease. However, its implementation is strongly limited by the chronic shortage of organ donors. To overcome this limitation, one solution is to regenerate the damaged kidney. In recent years, nanotechnologies have been used, combined with tissue engineering and material sciences, to develop promising applications in the field of organ regeneration. Different nanotechnological tools are being investigated for kidney regeneration, such as carbon nanotubes [16][17], exosomes [18][19], and nanofibers [20]. This field of nanomedicine is still in its infancy but is very promising. Nanoparticles and nanomaterials could be used to either deliver biomolecules into the kidney to promote regeneration or be used as a substrate to support kidney regrowth and regeneration.References
- Moss, D.M.; Siccardi, M. Optimizing Nanomedicine Pharmacokinetics Using Physiologically Based Pharmacokinetics Modelling. Br. J. Pharm. 2014, 171, 3963–3979.
- Scott, R.P.; Quaggin, S.E. Review Series: The Cell Biology of Renal Filtration. J. Cell Biol. 2015, 209, 199–210.
- Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv. Pharm. Bull. 2017, 7, 3–9.
- Lai, L.W.; Moeckel, G.W.; Lien, Y.H. Kidney-Targeted Liposome-Mediated Gene Transfer in Mice. Gene Ther. 1997, 4, 426–431.
- Shirai, T.; Kohara, H.; Tabata, Y. Inflammation Imaging by Silica Nanoparticles with Antibodies Orientedly Immobilized. J. Drug Target. 2012, 20, 535–543.
- Durigutto, P.; Sblattero, D.; Biffi, S.; De Maso, L.; Garrovo, C.; Baj, G.; Colombo, F.; Fischetti, F.; Di Naro, A.F.; Tedesco, F.; et al. Targeted Delivery of Neutralizing Anti-C5 Antibody to Renal Endothelium Prevents Complement-Dependent Tissue Damage. Front. Immunol. 2017, 8, 1093.
- Sutthasupha, P.; Lungkaphin, A. The Potential Roles of Chitosan Oligosaccharide in Prevention of Kidney Injury in Obese and Diabetic Conditions. Food Funct. 2020, 11, 7371–7388.
- Fu, Y.; Lin, Q.; Gong, T.; Sun, X.; Zhang, Z.-R. Renal-Targeting Triptolide-Glucosamine Conjugate Exhibits Lower Toxicity and Superior Efficacy in Attenuation of Ischemia/Reperfusion Renal Injury in Rats. Acta Pharm. Sin. 2016, 37, 1467–1480.
- Huang, C.; Zeng, T.; Li, J.; Tan, L.; Deng, X.; Pan, Y.; Chen, Q.; Li, A.; Hu, J. Folate Receptor-Mediated Renal-Targeting Nanoplatform for the Specific Delivery of Triptolide to Treat Renal Ischemia/Reperfusion Injury. ACS Biomater. Sci. Eng. 2019, 5, 2877–2886.
- He, X.; Yuan, Z.; Wu, X.; Xu, C.; Li, W. Low Molecular Weight Hydroxyethyl Chitosan-Prednisolone Conjugate for Renal Targeting Therapy: Synthesis, Characterization and in Vivo Studies. Theranostics 2012, 2, 1054–1063.
- Yang, B.; Gao, J.; Pei, Q.; Xu, H.; Yu, H. Engineering Prodrug Nanomedicine for Cancer Immunotherapy. Adv. Sci. 2020, 7, 2002365.
- Wischnjow, A.; Sarko, D.; Janzer, M.; Kaufman, C.; Beijer, B.; Brings, S.; Haberkorn, U.; Larbig, G.; Kübelbeck, A.; Mier, W. Renal Targeting: Peptide-Based Drug Delivery to Proximal Tubule Cells. Bioconjug. Chem. 2016, 27, 1050–1057.
- Kamaly, N.; He, J.C.; Ausiello, D.A.; Farokhzad, O.C. Nanomedicines for Renal Disease: Current Status and Future Applications. Nat. Rev. Nephrol. 2016, 12, 738–753.
- Tripathy, N.; Wang, J.; Tung, M.; Conway, C.; Chung, E.J. Transdermal Delivery of Kidney-Targeting Nanoparticles Using Dissolvable Microneedles. Cell Mol. Bioeng. 2020, 13, 475–486.
- Chen, H.-C.; Cheng, C.-Y.; Lin, H.-C.; Chen, H.-H.; Chen, C.-H.; Yang, C.-P.; Yang, K.-H.; Lin, C.-M.; Lin, T.-Y.; Shih, C.-M.; et al. Multifunctions of Excited Gold Nanoparticles Decorated Artificial Kidney with Efficient Hemodialysis and Therapeutic Potential. ACS Appl. Mater. Interfaces 2016, 8, 19691–19700.
- Murugesan, S.; Mousa, S.; Vijayaraghavan, A.; Ajayan, P.M.; Linhardt, R.J. Ionic Liquid-Derived Blood-Compatible Composite Membranes for Kidney Dialysis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 79B, 298–304.
- Reddy, A.R.N.; Reddy, Y.N.; Krishna, D.R.; Himabindu, V. Multi Wall Carbon Nanotubes Induce Oxidative Stress and Cytotoxicity in Human Embryonic Kidney (HEK293) Cells. Toxicology 2010, 272, 11–16.
- Herrera Sanchez, M.B.; Bruno, S.; Grange, C.; Tapparo, M.; Cantaluppi, V.; Tetta, C.; Camussi, G. Human Liver Stem Cells and Derived Extracellular Vesicles Improve Recovery in a Murine Model of Acute Kidney Injury. Stem Cell Res. Ther. 2014, 5, 124.
- Cantaluppi, V.; Medica, D.; Mannari, C.; Stiaccini, G.; Figliolini, F.; Dellepiane, S.; Quercia, A.D.; Migliori, M.; Panichi, V.; Giovannini, L.; et al. Endothelial Progenitor Cell-Derived Extracellular Vesicles Protect from Complement-Mediated Mesangial Injury in Experimental Anti-Thy1.1 Glomerulonephritis. Nephrol. Dial. Transplant. 2015, 30, 410–422.
- Mollet, B.B.; Bogaerts, I.L.J.; van Almen, G.C.; Dankers, P.Y.W. A Bioartificial Environment for Kidney Epithelial Cells Based on a Supramolecular Polymer Basement Membrane Mimic and an Organotypical Culture System. J. Tissue Eng. Regen. Med. 2017, 11, 1820–1834.
