Microneedling significantly enhanced transdermal delivery of both ALA and TMP in vitro. The MN hydrogel-forming system was comparable with the MN dissolving system for ALA delivery (~3000 nmol/cm2over 6h), however superior for delivery of the much larger TMP molecule (~14 nmol/cm2over 24h, compared to 0.15 nmol/cm2). Thus, these results have opened the potential for investigating microneedling with many other PSs.
- photodynamic therapy
- drug delivery
- transdermal
- cutaneous
- cancer
1. Introduction
Historically, phototherapy can trace its roots to Ancient Egyptian, Chinese, and Indian civilizations. Light combined with photosensitizing natural formulations were used to treat illnesses such as vitiligo, psoriasis, and skin cancer. In 1900, Raab and von Tappeiner described the phenomena of cell death induced by a combination of chemicals (acridine dye) and light on the protozoa paramecia ( Figure 1 ) [8][1]. Further investigations gave birth to the revolutionary Photodynamic Action . The first biomedical use of this innovation was reported by Friedrich Meyer-Betz, in 1912, who self-injected hematoporphyrin, resulting in pain and swelling on light-exposed areas [9][2]. Subsequently, in 1961, Lipson et al. demonstrated that hematoporphyrin derivatives (HpD) accumulated in tumors and emitted fluorescence could be applied as a diagnostic tool [10][3].
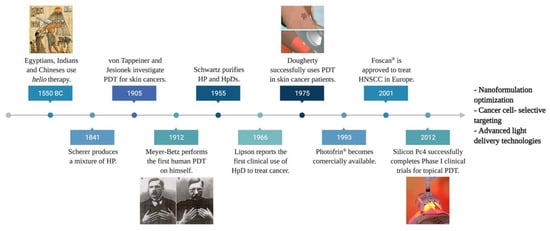
Further, Dougherty et al. introduced PDT in the 1970s by observing complete mammary tumor remission in vivo using HpD combined with the red light [11][4]. A subsequent clinical study using 25 patients showed complete response in 98 out of 113 skin tumors, partial response in 13, and only two resistant tumors [12][5]. These findings led to the first approval of the PDT drug, Photofrin ® , to treat bladder cancer in Canada in 1993 [13][6]. In 2001, Foscan ® became the first second-generation PS agent commercially available for PDT, especially in head-and-neck squamous cell carcinoma (HNSCC) treatment. Ultimately, in 2012, silicon phthalocyanine (Pc) 4 entered a phase I clinical trial as the first topical PDT agent ( Figure 1 ) [14][7]. Currently, PDT is employed to treat a wide range of diseases, such as leishmaniasis, psoriasis, neovascular macular degeneration, cardiology, urology, immunology, ophthalmology, dentistry, and dermatology [15][8].
The distinctiveness of PDT relies on its highly selective mechanism of action. Utilizing two independently nontoxic components (i.e., a PS and light) to produce cytotoxicity within a tumor. There is a high level of tumor selectivity to PS due to increased tumor vasculature surface area, higher membrane permeability of cancer cells, and decreased lymphatic drainage [16][9]. An ideal PS remains inert until a light source is focused onto the intended area to “activate” the PS, boosting its selectivity over surrounding healthy tissues. Ultimately, a third intrinsic component for PDT, molecular oxygen, present in tissue’s extracellular and intracellular spaces, serves as the substrate for ROS formation. The generation of singlet oxygen and superoxide anions results in tumor cytotoxicity as both can directly react with and damage biomolecules such as lipids, proteins, and nucleic acids [17][10].
Several studies have demonstrated PDT as a viable treatment option against early-stage esophageal dysplasia, lung, HNSC, anal, bladder, peritoneal ovarian, and non-melanoma skin cancers (NMSC) [18][11]. Despite the encouraging clinical results of PDT, some PSs themselves have been reported to have prolonged skin phototoxicity, low lesion selectivity, hydrophobic nature, aggregation proneness, poor bioavailability, high-dose requirements, adverse side effects, off-targeting, and development of drug resistance [19,20,21][12][13][14]. The use of drug delivery systems (DDS) to overcome these shortcomings has been examined. In this context, PSs can be ideally delivered to therapeutic action sites while reducing adverse side effects [21][14]. Among DDS, the transdermal route stands out for dermatological applications. However, the inherent protective epidermic layer, the high molecular weight of some PSs (>500 Daltons), and extremes of polarity remain a challenge for crossing the skin barrier [22][15].
2. Transdermal Drug Delivery of PDT Agents
The skin barrier is a lipid layer along which individual molecules can migrate through diffusion. Although skin has a large surface area, transdermal drug delivery is challenging since it acts as a formidable barrier. Molecules can enter through keratinocytes (the transcellular pathway), the lipid matrix occupying the intercellular spaces (the intercellular pathway), hair follicles, sebaceous glands, or sweat glands (the transappendageal pathway) [23][16]. The process is controlled mainly by the molecule’s permeant hydrophilicity, size, and hydrogen-bonding ability [24][17]. Consequently, substances with molecular weight lower than 600 Da, water/octanol partition coefficient (logP values) in the range of 1–3, and those with a low melting point can be more easily absorbed into the skin. In contrast, larger molecules and highly polar compounds cannot pass the cutaneous barrier [25][18].
From the penetrant perspective, the skin behaves like a mechanical nanoporous barrier perforated by many gap-like or quasi-semicircular pathways. Previous studies estimate an average passage-width range of 0.4 to 36 nm. Arguably, pore-size distribution is non-symmetrical, with a peak around 20 nm and a steeper decline on the transbarrier pathways’ low-sized side. Moreover, existing data demonstrate that the critical factor for hydrophilic entity motion through the skin is primarily molecular size and not molecular weight [26][19]. Thus, several parallel physiological paths between two cells increase the skin porosity, which is crucial for topical drug delivery.
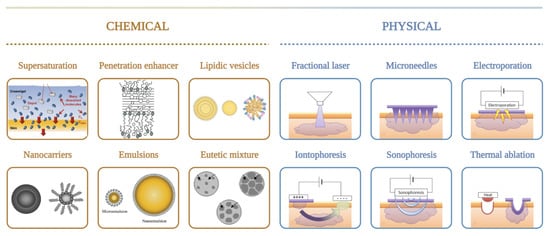
One extensively investigated strategy to enhance drug delivery through the skin is by adding chemical enhancers such as fatty acids, surfactants, esters, alcohols, pyrrolidones, amines, amides, sulphoxides, terpenes, alkanes, and phospholipids. Conversely, mechanical, physical, and active transport techniques, such as microneedles (MNs), jet injectors, iontophoresis, ultrasound, electroporation, photomechanical waves, magnetophoresis, laser radiation, and skin abrasions, are also available to enhance skin penetration [25][18]. The use of chemical enhancers is restricted due to the limited permeability of macromolecules, while physical approaches are invasive and can damage the skin barrier properties ( Figure 2 ). To overcome some limitations of chemical and physical enhancers, nanoparticles are being developed to improve the absorption and transdermal drug release in a controlled manner for a prolonged period.
The application of nanotechnology for conventional PSs has helped to overcome many shortcomings, particularly related to the hydrophobic nature of these compounds, which limits the penetration through the skin and cell membranes [20,27][13][20]. While hydrophobic PSs, such as phthalocyanines (Pcs) and chlorins, form aggregates in physiological solutions [28][21], hydrophilic PSs raise interaction problems with biological tissues. In this context, liposomes have been reported as suitable delivery systems for carrying both hydrophilic and hydrophobic PSs and leading to improved clinical applications [29][22]. For instance, the extensively studied PS, 5-aminolevulinic acid (ALA), is not internalized well by cells and has no specificity for diseased tissues [27][20]. Therefore, PSs associated with nanoparticles have evolved to avoid self-aggregation and improve delivery to the target site.
3. Chemical Approaches for Transdermal Delivery
Furthermore, Primo et al. reported the synthesis and in vitro characterization of magnetic NE with mTHPC. Their results suggested that magnetic NE improves the penetration of mTHPC in skin layers leading to an adequate accumulation in vivo . The retention studies showed that the PS concentration in deep tissue layers was significantly higher in the presence of magnetic nanoparticles, making possible its topical application in skin cancer PDT protocols and hyperthermia activation in synergic procedures [43][23].
Although the superiority of liposomal PSs was not initially demonstrated [48][24], in 2001, Pierre et al. proposed modified liposomes having lipid composition similar to the mammalian SC to optimize ALA transdermal delivery in skin cancers [49,50][25][26]. The liposomal vesicles containing 5.7% ALA showed higher skin retention ( p < 0.05) on the epidermis with a decrease of skin permeation compared to an aqueous solution [50][26]. Likewise, ALA liposomal formulations resulted in prolonged ALA-induced PpIX accumulation, as well as better epidermal targeting [51][27]. Additionally, enhanced ALA penetration with hydrophobic derivatives of ALA (ALA-esters, ALA-amino acid derivatives, and ALA dendrimers) showed promising results in vitro [52][28]. However, although some ALA-esters yielded strong in vivo results, only the MAL formulation is currently used in cutaneous malignancies [34][29].
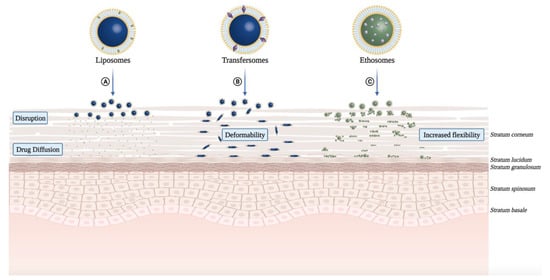
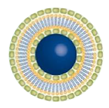
More recently, cationic ALA-loaded ultra-deformable liposomes, also known as transfersomes ( Figure 3 B), showed higher stability and permeability than other tested liposomal formulations, enabling ALA delivery to deeper skin layers [49][25] ( Table 1 ). Moreover, cationic transfersomes allowed higher retention of ALA in cells and improved the induction of PpIX, indicating better photosensitizing properties compared to other hybrid lipoidal vesicles.
| Types | Nano-/Micro-Emulsions | Conventional Liposomes |
Niosomes | Transfersomes | Ethosomes | Invasomes |
|---|---|---|---|---|---|---|
| Structure |  |
 |
 |
 |
 |
 |
| Timeline | 1961 | 1965 | 1970 | 1991 | 1997 | 2004 |
| Composition | Oil or main oleaginous ingredient Surfactant/emulsifier Water |
Phospholipids and cholesterol | Non-ionic surfactants 5–10% Other additives Buffer QS |
Lipid 5% w/v Edge activator 1% w/v Buffer QS |
Phospholipid 5–10% w/w Ethanol 20-50% w/w Buffer QS |
Soy-phosphatidylcholine 10% w/w and Lyso soy-phosphatidylcholine 0.7% Ethanol 10% w/w Terpenes 1% w/w Buffer QS |
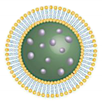
| Characteristics | A liquid colloidal dispersion system which is kinetically stable, with a droplet size <100 nm | Microscopic spheres | Microscopic spheres | Ultra-deformable liposome | Elastic liposome | Ultraflexible and Elastic liposome |
| Flexibility | - | Rigid | Rigid | High deformability due to edge activator (surfactant) | High deformability and elasticity due to ethanol | High deformability and elasticity |
| Permeation Mechanism | Diffusion/Fusion/Lipolysis | Diffusion/Fusion/Lipolysis | Diffusion/Fusion/Lipolysis | Deformation | Lipid perturbation | Deformation and Lipid perturbation |
| Trademarks of topical photosensitizers | 5-ALA Ameluz® | Lipoxala® spray, mTHPC Foslip®, BPD-MA Visudyne®, ZnPc CGP55847® BPD-MA | - | mTHPC Fospeg® | - | - |
Dragicevic-Curic et al. also evaluated the in vitro skin penetration of mTHPC-loaded neutral, anionic, and cationic transfersomes using human abdominal skin mounted in Franz diffusion cells ( Figure 3 B). Besides the effect of surface charge of transfersomes on skin penetration of mTHPC, its impact on physical properties (particle size, polydispersity index, lamellarity) and the physicochemical stability of vesicles were investigated ( Table 1 ). From these mostly unilamellar and spherical vesicles, cationic transfersomes possessed the highest penetration enhancing ability (mTHPC -amount delivery to SC and deeper skin layers) than conventional liposomes, neutral, and anionic transfersomes. Regarding stability, contrasting to anionic transfersomes, neutral and cationic transfersomes were stable for 9 months at 4 °C. Thus, mTHPC-loaded cationic transfersomes showed the highest potential to be used in anticancer PDT [66][30].
4. Physical Approaches for Transdermal Delivery
The controlled disruption and ablation of the SC, the predominant barrier for topical drug delivery, can be achieved via microneedling, radiofrequency (RF), and lasers. Recently, the concept of using a laser to treat the skin has attracted increasing attention. Laser-assisted drug delivery (LADD) involves controlled, selective destruction of the epidermis and dermis to allow for penetration and absorption of topical medications and large drug molecules. Challenges in predicting LADD’s efficacy and safety include: the unpredictability of drug dosing and possible systemic toxicity, variability in absorption, induction of localized and systemic hypersensitivity, and inconsistencies in treatment protocol [76][31] ( Figure 54 ).
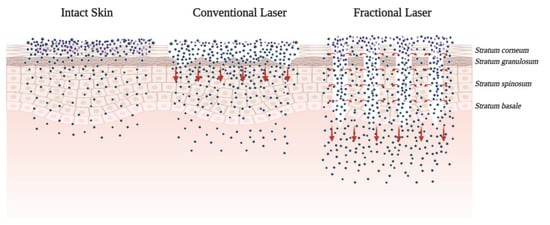
However, although the positive results of LADD-ALA-PDT were observed, a comparative study by Chen et al. to evaluate ALA-PDT revealed that plum-blossom needling (a method of shallow insertion of multiple needles into the skin) had more broad diffusion of ALA than the CO 2 AFXL while having a similar clinical effect at a much lower cost. A clinical trial also revealed that the surface fluorescence intensity was stronger in needle-pretreated-lesion than in laser-pretreated-lesion. It appears that plum-blossom needling treatment may be clinically superior for enhancing ALA-tDDS and other topical skin medications [86][32].
The parenteral administration of near-IR preformed PSs suffers from low selectivity and may result in prolonged skin photosensitivity. MNs can provide localized drug delivery to skin lesions and overcome the limitations of delivering into the dermal layer. In superficial cancer treatments, topical drug administration faces severely low transfer efficiency ( Figure 65 ). MN-based systems have achieved excellent administration capabilities and have been tested for pre-clinical PDT [87][33].
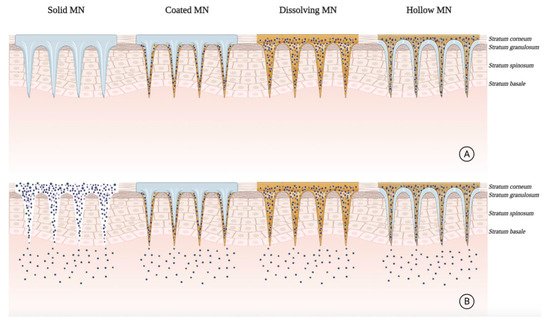
Intradermal delivery of the preformed near-IR PS: 5,10,15,20-tetrakis (2,6-difluoro-3-N-methyl-sulfamoylphenyl bacteriochlorin (Redaporfin TM ) using dissolving MNs was successful in vitro and in vivo to treat nodular BCC. MNs demonstrated complete dissolution 30 min after topical application and revealed sufficient mechanical strength to penetrate 450 μm depth of the skin. In vitro studies illustrated that the drug delivery and detection was 5 mm in-depth of the skin. In vivo biodistribution studies in athymic nude mice showed both fast initial release with localized drug delivery. The MN-treated mice showed a progressive decrease in the PS at the application site over 7 days. However, most of the skin surface showed fluorescence levels comparable to those seen in the negative control group. These results suggested beneficial effects for polymeric MN arrays in minimally invasive intradermal delivery to enhance PDT in deep skin lesions [97][34].
References
- Berg, K.; Selbo, P.K.; Weyergang, A.; Dietze, A.; Prasmickaite, L.; Bonsted, A.; Engesaeter, B.O.; Angell-Petersen, E.; Warloe, T.; Frandsen, N.; et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J. Microsc. 2005, 218, 133–147.
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387.
- Lipson, R.L.; Baldes, E.J.; Olsen, A.M. The use of a derivative of hematoporhyrin in tumor detection. J. Natl. Cancer Inst. 1961, 26, 1–11.
- Dougherty, T.J.; Grindey, G.B.; Fiel, R.; Weishaupt, K.R.; Boyle, D.G. Photoradiation therapy. II. Cure of animal tumors with hematoporphyrin and light23. J. Natl. Cancer Inst. 1975, 55, 115–121.
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635.
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19.
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 1–8.
- Rkein, A.M.; Ozog, D.M. Photodynamic Therapy. Dermatol. Clin. 2014, 32, 415–425.
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107.
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in modern photodynamic therapy of cancer: A review of cellular resistance patterns affecting the therapeutic response. Pharmaceutics 2020, 12, 632.
- Monge-Fuentes, V.; Muehlmann, L.A.; De Azevedo, R.B. Perspectives on the application of nanotechnology in photodynamic therapy for the treatment of melanoma. Nano Rev. 2014, 5, 24381.
- D’Alessandro, S.; Priefer, R. Non-porphyrin dyes used as photosensitizers in photodynamic therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 101979.
- Muehlmann, L.A.; Joanitti, G.A.; Silva, J.R.; Longo, J.P.F.; Azevedo, R.B. Liposomal photosensitizers: Potential platforms for anticancer photodynamic therapy. Braz. J. Med. Biol. Res. 2011, 44, 729–737.
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 1–19.
- Morrow, D.I.J.; Mccarron, P.A.; Woolfson, A.D.; Donnelly, R.F. Innovative strategies for enhancing topical and transdermal drug delivery. Open Drug Deliv. J. 2007, 1, 36–59.
- Supe, S.; Takudage, P. Methods for evaluating penetration of drug into the skin: A review. Ski. Res. Technol. 2021, 27, 299–308.
- Keservani, R.K.; Bandopadhyay, S.; Bandyopadhyay, N.; Sharma, A.K. Design and fabrication of transdermal/skin drug-delivery system. In Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–178.
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259.
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004, 56, 675–711.
- Chen, B.; Pogue, B.W.; Hasan, T. Liposomal delivery of photosensitising agents. Expert Opin. Drug Deliv. 2005, 2, 477–487.
- Damoiseau, X.; Schuitmaker, H.J.; Lagerberg, J.W.M.; Hoebeke, M. Increase of the photosensitizing efficiency of the Bacteriochlorin a by liposome-incorporation. J. Photochem. Photobiol. B Biol. 2001, 60, 50–60.
- Derycke, A.S.L.; de Witte, P.A.M. Liposomes for photodynamic therapy. Adv. Drug Deliv. Rev. 2004, 56, 17–30.
- Primo, F.L.; Michieleto, L.; Rodrigues, M.A.M.; Macaroff, P.P.; Morais, P.C.; Lacava, Z.G.M.; Bentley, M.V.L.B.; Tedesco, A.C. Magnetic nanoemulsions as drug delivery system for Foscan®: Skin permeation and retention in vitro assays for topical application in photodynamic therapy (PDT) of skin cancer. J. Magn. Magn. Mater. 2007, 311, 354–357.
- Casas, A.; Fukuda, H.; Di Venosa, G. The influence of the vehicle on the synthesis of porphyrins after topical application of 5-aminolaevulinic acid. Implications in cutaneous photodynamic sensitization. Br. J. Dermatol. 2000, 143, 564–572.
- Oh, E.K.; Jin, S.-E.; Kim, J.-K.; Park, J.-S.; Park, Y.; Kim, C.-K. Retained topical delivery of 5-aminolevulinic acid using cationic ultradeformable liposomes for photodynamic therapy. Eur. J. Pharm. Sci. 2011, 44, 149–157.
- Pierre, M.B.R.; Tedesco, A.C.; Marchetti, J.M.; Bentley, M.V.L. Stratum corneum lipids liposomes for the topical delivery of 5-aminolevulinic acid in photodynamic therapy of skin cancer: Preparation and in vitro permeation study. BMC Dermatol. 2001, 1, 1–6.
- Tsai, J.-C.; Chen, I.-H.; Wong, T.-W.; Lo, Y.-L. In vitro/in vivo correlations between transdermal delivery of 5-aminolaevulinic acid and cutaneous protoporphyrin IX accumulation and effect of formulation. Br. J. Dermatol. 2002, 146, 853–862.
- Perotti, C.; Fukuda, H.; Divenosa, G.; Macrobert, A.J.; Batlle, A.; Casas, A. Porphyrin synthesis from ALA derivatives for photodynamic therapy. In vitro and in vivo studies. Br. J. Cancer 2004, 90, 1660–1665.
- Casas, A.; Batlle, A. Aminolevulinic acid derivatives and liposome delivery as strategies for improving 5-aminolevulinic acid-mediated photodynamic therapy. Curr. Med. Chem. 2006, 13, 1157–1168.
- Dragicevic-Curic, N.; Scheglmann, D.; Albrecht, V.; Fahr, A. Development of liposomes containing ethanol for skin delivery of temoporfin: Characterization and in vitro penetration studies. Colloids Surf. B Biointerfaces 2009, 74, 114–122.
- Ibrahim, O.; Wenande, E.; Hogan, S.; Arndt, K.A.; Haedersdal, M.; Dover, J.S. Challenges to laser-assisted drug delivery: Applying theory to clinical practice. Lasers Surg. Med. 2018, 50, 20–27.
- Chen, J.; Zhang, Y.; Wang, P.; Wang, B.; Zhang, G.; Wang, X. Plum-blossom needling promoted PpIX fluorescence intensity from 5-aminolevulinic acid in porcine skin model and patients with actnic keratosis. Photodiagn. Photodyn. Ther. 2016, 15, 182–190.
- Li, D.; Hu, D.; Xu, H.; Patra, H.K.; Liu, X.; Zhou, Z.; Tang, J.; Slater, N.; Shen, Y. Progress and perspective of microneedle system for anti-cancer drug delivery. Biomaterials 2021, 264, 120410.
- Hamdan, I.M.N.; Tekko, I.A.; Matchett, K.B.; Arnaut, L.G.; Silva, C.S.; McCarthy, H.O.; Donnelly, R.F. Intradermal delivery of a near-infrared photosensitizer using dissolving microneedle arrays. J. Pharm. Sci. 2018, 107, 2439–2450.
