Alaria alata flukes are cosmopolitan parasites. In Europe, the definitive hosts are red foxes (Vulpes vulpes), wolves (Canis lupus), and raccoon dogs (Nyctereutes procyonoides), as well as animals that belong to the Felidae family. Intermediate hosts, such as snails and frogs, are the sources of infection for definitive hosts. The developmental stages of A. alata mesocercariae may occur in paratenic hosts, including many species of mammals, birds, and reptiles, as well as in wild boars (Sus scrofa), which are important from the zoonotic point of view. Alaria alata is a widespread trematode that is considered a potential cause of a human disease called alariosis, which is associated with the consumption of raw or undercooked meat of intermediate or paratenic hosts of this parasite.
- Alaria alata
- parasite
- risk
- meat
- venison
- pork
1. Characteristics of A. alata

The adult stage of
isolated from the small intestine of a red fox; 40× magnification (by J. Karamon).
The larval stage of
has the shape of an oval, reaches up to 0.5 mm in length, and has fine parallel lines. It is equipped with a mouth and abdominal sucker [15] (
). In several studies, a series of electron microscopy photos of larval forms originating from wild boars revealed additional sucker-like surface structures.
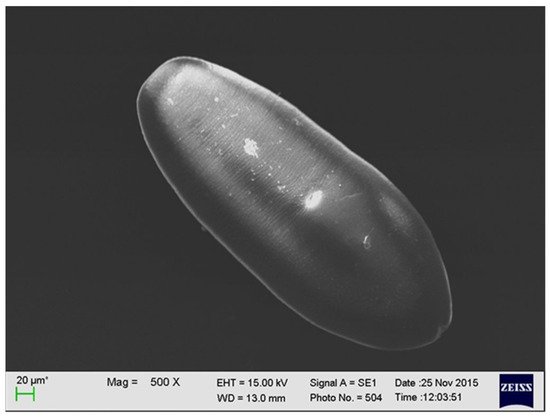
The larval stage of
detected in the muscle tissue of a wild boar (by M. Wasiak).
2. Life Cycle of A. alata
The life cycle of the
genus is complex and involves definitive, intermediate, and paratenic hosts (
). The definitive hosts are carnivores, including foxes, wolves, raccoons, lynxes, martens, badgers, dogs, and cats [16][17][18]. They become infected by eating frogs or tadpoles that contain mesocercariae, whose length can reach up to 0.5 mm (
). These parasites migrate through abdominal and thoracic cavities or via the circulation to the lungs, where the mesocercariae enter the metacercarial stage. Next, they are swallowed and develop in the small intestine, reaching 3–6 mm in length and 1–2 mm in breadth as adult flukes [15]. In studies on the distribution of the parasites in the small intestines of foxes,
were detected mostly in the anterior parts of the intestines in almost all infected foxes (99.4%) [19]. Eggs (oval, size 98–125 × 62–81, light brown and operculated with a lid–operculum), which are a dispersive form of the parasite, are laid in definitive hosts and excreted with the feces into the environment. Miracidia, an invasive form of the parasite in intermediate hosts, are released from the eggs in aquatic environments, and they infect the first intermediate hosts, which are freshwater snails (e.g.,
,
spp.). The miracidia develop into sporocysts that hatch a fast-moving larval stage—cercariae—which leave the snails, penetrate tadpoles or frogs, and develop into non-reproductive mesocercariae. The consumption of these amphibians by carnivores completes the life cycle of
. However, as mentioned before, the life cycle of this parasite can also involve paratenic hosts, such as wild boars, mice, rats, martens, polecats, and pigs, as well as wild birds and some species of snakes and lizards. Similarly to the definitive hosts, they can be infected by eating mesocercariae from intermediate (tadpoles or frogs) or other paratenic hosts [20][17][18]. Within these hosts, the mesocercariae do not reach the stage of adult flukes; however, they can survive for months in the connective tissues between muscles or the adipose tissue, which constitute a kind of reservoir of this fluke for definitive hosts or other paratenic hosts. The migration of the mesocercariae from one paratenic host to another does not reduce the infectivity of the parasite [15]. In addition, humans can become paratenic hosts by consuming mesocercariae that are present in raw or undercooked game, pork, frog legs, or snails [21]. Infections with
in humans cause alariosis.
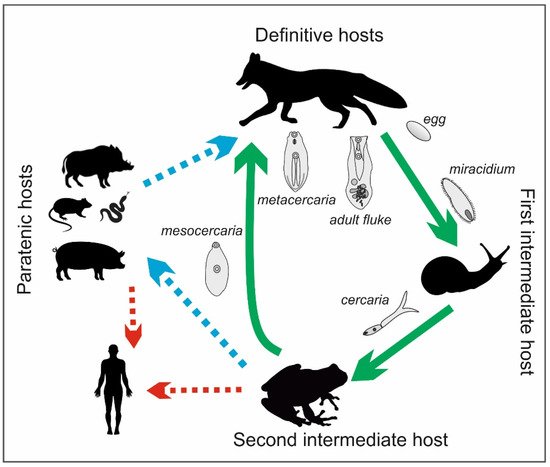
Life cycle of
(by J. Karamon).

movement sequence in a muscular cyst of a wild boar (by M. Różycki).
3. Detection of A. alata in Definitive and Paratenic Hosts
The presence of adult flukes in definitive hosts is mainly connected with the microscopic detection of eggs in fecal samples or post-mortem parasite recovery from intestinal content. However, the diagnosis of
infection in the meat of paratenic hosts is performed during inspections for
spp. Therefore, most reports of the presence of
in wild boar meat in the literature come from tests for the presence of
spp. using the reference magnetic stirrer method with artificial digestion (MSM) according to ISO 18743 [22]. This technique is not dedicated to the detection of flukes of
spp. In 2006, the German Federal Institute for Risk Assessment pointed out the existence of a risk of infection with alariosis for game consumers [23]. At that time, because of the lack of an appropriate method for detecting this parasite, the real number of infected wild boars could not be determined. Therefore, in 2010, the mesocercaria migration technique (AMT) was developed for the detection of
. In the AMT, a sample of minced meat weighing 30 ± 2 g is transferred to a strainer, which is placed in a funnel and immersed in warm water (46–48 °C). After 90 min, 20 mL of the liquid is drained into a measuring cylinder and then into a Petri dish or larval counting basin, and it is viewed under a stereomicroscope or trichinoscope at a magnification of 15–20× [15]. Subsequently, German researchers used the method described above to examine archived wild boar meat samples that were classified as not containing
mesocercariae during the detection of
spp. with the artificial digestion method. The AMT showed that 11.5% of the samples tested contained
mesocercariae [21]. The limited possibility of detecting
mesocercariae using artificial digestion was caused by their lower resistance to the digestive fluid (HCL/pepsin), which damaged the parasites and caused the loss of their characteristic mobility, making their identification difficult. Due to their characteristic shape and size,
mesocercariae often do not reach the final stage of the MSM (examination of sediments) because they stay in the strainer. Therefore, one of the reasons for the low detection of mesocercariae with the MSM may also be the inadequate diameter of the mesh size in the sieves used for the tests [24]. However, according to ISO 18743:2015, which has been indicated as a reference method in the EU Commission Regulation 2020/1478 since October 2020, the diameter of the mesh size may vary from 180 to 200 µm. A larger mesh size may provide a greater possibility for the detection of
mesocercariae. Moreover, these parasites are most often located in the layer of connective tissue between the muscles, especially where there is a large amount of adipose tissue; however, for MSM testing for trichinosis, it is advised that samples are free of fat and fascia [25]. As a result, in official statistics, the presence of
might be underreported. Studies were also conducted in Latvia to determine the level of potentially false-negative results found using the MSM compared with the level in the results obtained using the AMT. In these analyses, it was found that 40% of the samples tested with the MSM contained
mesocercariae, but after using the AMT, this percentage increased to 76.7%. In addition, a significant difference in the number of mesocercariae detected was observed. Their number per gram ranged from 0.02 to 1.22 when using the AMT and from 0.02 to 0.56 when using the MSM. In 13 (21.7%) samples, mesocercariae were found only on the sieve during the ASM test. This number indicates that these samples were false negatives when tested with the MSM [24]. In studies performed in the Czech Republic between 2012 and 2013 with the AMT, the percentage of wild boar meat samples containing
mesocercariae was 6.8%; however, when using the MSM, no mesocercariae were found in any of the 221 samples tested [26]. In Lithuania, during routine tests for the detection of
spp., the percentage of simultaneously detected samples containing
mesocercariae was 7% [24]. In Poland, veterinary inspectors observed the presence of
during regulatory testing of wild boar carcasses for trichinellosis. A questionnaire was sent to participants in proficiency tests for
spp. detection in Poland in 2020 (489 participants), which revealed the occurrence of mesocercariae in wild boar meat samples at the level of 4%. According to the data obtained,
were found only in wild boars. However, according to data collected by the Veterinary Research Institute in Poland, 33% of the wild boars inhabiting the southeastern region of Poland (which is rich in water reservoirs) were infected with mesocercariae (unpublished data).
4. A. alata as a Potential Threat in the Production of Food of Animal Origin and Preventive Actions
Humans may act as paratenic hosts; in some countries, depending on local dietary habits, they can be infected by eating frogs (frog legs) or any predators of frogs, among which the wild boar is the main source of infection [27]. Frog-eating birds (herons, birds of prey, etc.) must also be taken into account as a source of human infection, even though these are not very popular dishes and are not normally consumed. There are also other sources of infection, but they are highly unlikely; these include Mustelidae (badgers, weasels, otters, etc.), Procyonidae (raccoons and coatis), which have been found to harbor the mesocercarial stage in their tissues, and even reptiles [28][29][30]. The human hazards related to the consumption of meat products that contain mesocercariae of
depend on various factors, such as prior freezing of the meat, the amount of meat consumed, and the methods used in the processing of the meat [31]. Freezing is recommended for inactivating many parasites, including
or
[32]. Gonzales-Fuentes et al. (2015) pointed out that freezing game meat to an internal temperature of at most −13.7 °C inactivates mesocercariae [33]. The survival of the larvae of
at the temperatures in refrigerators (4 to 8 °C) is very high, even during long-term storage; therefore, the potential risk for consumers remains high [34]. To date, there is no precise information about the doses required for infections. However, after analyzing confirmed alariosis cases, it can be assumed that the severity of the symptoms is correlated with the number of larvae taken in [35][36][37][38]. According to current knowledge, heat treatment is the most effective method for the inactivation of
mesocercariae in wild boar meat. Heating at 72 °C for 2 min kills mesocercariae; therefore, the meat becomes fit for consumption [39]. In the work of Portier et al. (2011), it was shown that
larvae could survive for at least five days when frozen (−18 °C) [40]. The most effective method for killing these flukes—as in the case of
spp.—is cooking at 71 °C [25]. In addition, hygienic production is very important for minimizing the risks to consumers, as smear infections can occur during meat processing [35][36][41][38]. It is difficult to estimate the risk linked with the consumption of raw or undercooked meat products made from organic or free-range pigs. Studies conducted in Serbia on diaphragm samples collected from 72 free-range pigs showed that the percentage of samples infected with
mesocercariae was 2.77%. The researchers then underlined that the risk of human alariosis increases in regions where there is a tradition of making homemade pork products [42]. In addition, in other countries, delicacies made from raw ground pork are very popular dishes. Among them, there are types of fresh sausages, such as Italian sausage, bratwurst, Polish steak tartare, and German
. These products are made from chopped, ground, or even pureed uncooked pork meat. In some territories, such as France and some Nordic countries, the consumption of game meat is related to the historical culture, in which this type of meat is shared among hunters and their families. Therefore, this group of consumers is especially exposed to the consumption of meat infected by
[23][34]. In 2014, in Germany, studies were performed to determine the survival rate of
larvae during the production of raw cured meat products, such as raw ham, salami, and raw sausage. These studies intended to clarify whether mesocercariae are eliminated during the production of these products and if traditional meat products play a role as sources of
infections in humans. In the experiment, the meats of wild boars and raccoons that were positive for the presence of
were used. A comparison of the three different technological processes showed that no live larvae were found in any of the ready-made hams, which proved that 100% of
mesocercariae were inactivated during production. However, 11.9% of salami sausages and 18.2% of the second type of raw sausage contained mesocercariae 24 h after preparation in the initial fermentation stage. Therefore, even tasting the meat during production may lead to an intake of
mesocercariae. These results indicate that the consumption of raw sausages in particular may be risky for consumers, especially if these products are consumed immediately after production [43]. The German Federal Institute for Risk Assessment (BfR) conducted an evaluation to determine the risk of infection with parasites after consumption of game meat. This type of product is generally consumed in low amounts in Germany (200 to 400 g per person each year). However, the consumption of game meat in Germany has increased in recent years, and a certain group of people, including hunters, their relatives, and their friends, can consume 50–90 times more meals containing game each year [44][45][46]. There is also an increasing interest in medium or rare game meat, which is pink at the core. The document mentioned above includes a recommendation to thoroughly cook game meat, raw game sausages, and raw meat products before consumption [39].
References
- Duncker, H.C.J. Distomeen in Schweinefleisz. Zschr mikr. Fleischschau 1881, 2, 22–24.
- Duncker, H.C.J. Muskel-Distomeen. Zschr mikr. Fleischschau 1881, 2, 141.
- Duncker, H.C.J. Distomeen im Schweinefleisch. Zschr mikr. Fleischschau 1884, 3, 39–42.
- Duncker, H.C.J. Die Muskel-Distomeen. Berl. Münchener Tierärztliche Wochenschr. 1896, 24, 279–282.
- Duncker, H.C.J. Die Muskel-Distomeen. Zschr Fleisch. Milchhyg. 1897, 7, 197–198.
- Bugge, G. Der muskelegel Dunckers beim frosch. Zschr Fleisch. Milchhyg. 1942, 54, 73–76.
- Stefański, W.; Tarczyński, S. Sur le developpement de l’Agamodistomum suis Duncker, 1881. Acta Parasitol. Pol. 1953, 1, 149–154.
- Bosma, N.J. Alaria mustelae sp. nov., a trematode requiring four hosts. Science 1931, 74, 521–522.
- Olivier, L.; Odolaug, T.O. A new mesocercaria (Trematoda: Strigeata) with a note on its further developement. J. Parasitol. 1938, 24, 369–374.
- Johnson, A.D. Life history of Alaria marcianae (La Rue, 1917) Walton, 1949 (Trematoda: Diplostomatidae). J. Parasitol. 1968, 54, 324–332.
- Compton, T.L. Alaria arisaemoides Augustine and Uribe, 1927 (Strigeidae) from interior Alaska. Can. J. Zool. 1969, 47, 1420–1421.
- Pearson, J.C. Studies on the life cycles and morphology of the larval stages of Alaria arisaemoides Augustine and Uribe, 1927 and Alaria canis La Rue and Fallis, 1936 (trematode: Diplostomidae). Can. J. Zool. 1956, 34, 295–387.
- Sekerak, A.D. Alaria taxideae Swanson and Erickson, 1946 in pine marten from central Alaska (Trematode: Diplostomatidae). Can. J. Zool. 1969, 47, 266.
- Nacheva, L.V.; Manikovskaya, N.S. Structure of some strigeatas’ organ of Brandes. In Proceedings of the Sbornik Nauchnykh Stateĭ po Materialam Mezhdunarodnoĭ Nauchnoĭ Konferentsii, Teoriya i Praktika Bor’by s Parazitarnymi Boleƶnyami, Posvyashaetsya 90-Letiyu so Dnya Rozhdeniya Andreya Stefanovicha Bessonova, Vypusk 20, Moscow, Russia, 15–17 May 2019; pp. 399–403.
- Möhl, K.; Grosse, K.; Hamedy, A.; Wüste, T.; Kabelitz, P.; Lücker, E. Biology of Alaria spp. and human exposition risk to Alaria mesocercariae—A review. Parasitol. Res. 2009, 105, 1–15.
- Takeuchi-Storm, N.; Al-Sabi, M.N.S.; Thomsborg, S.M.; Enemark, H.L. Alaria alata Mesocercariae among Feral Cats and Badgers, Denmark. Emerg. Infect. Dis. 2015, 21, 1872–1874.
- Wójcik, A.R.; Grygon-Franckiewicz, B.; Żbikowska, B. Current data of Alaria alata (Goeze, 1782) according to own studies. Vet. Med. 2002, 58, 517–519.
- Chmurzyńska, E.; Różycki, M.; Bilska-Zając, E.; Karamon, J.; Cencek, T. Alaria alata—Potential threat for humans, prevalence and diagnostic measures. Vet. Life 2013, 88, 780–784.
- Karamon, J.; Sroka, J.; Dąbrowska, J.; Bilska-ając, E.; Skrzypek, K.; Różycki, M.; Zdybel, J.; Cencek, T. Distribution of parasitic helminths in the small intestine of the red fox (Vulpes vulpes). Pathogens 2020, 9, 477.
- Wasiluk, A. Alaria alata infection—Threating yet rarely detected trematodiasis. J. Lab. Diag. 2013, 49, 33–37.
- Riehn, K.; Hamedy, A.; Grosse, K.; Wüste, T.; Lücker, E. Alaria alata in wild boars (Sus scrofa, Linnaeus, 1758) in the eastern parts of Germany. Parasitol. Res. 2012, 111, 1857–1861.
- Rossi, P.; de Smet, K.; Pozio, E. Detection of Trichinella Larvae in Meat: Comparison of ISO 18743:2015 with Regulation (EU) 2015/1375. Food Anal. Methods 2017, 10, 634–639.
- Anonymous. Wid Boar Meat Can Contain Duncker’s Muscle Fluke, 2017 (Updated BfR Opinion No. 011/2017). BfR. Available online: https://mobil.bfr.bund.de/cm/349/wild-boar-meat-can-contain-Dunckers-muscle-fluke.pdf (accessed on 12 July 2021).
- Ozoliņa, Z.; Deksne, G. Effectiveness of two methods for mesocercariae of Alaria alata detection in wild boars (Sus scrofa). EEB 2017, 15, 25–28.
- Gamble, H.R.; Bessonov, A.S.; Cuperlovic, K.; Gajdhar, A.A.; van Knapen, F.; Noeckler, K.; Schenone, H.; Zhu, X. International Commission on Trichinellosis: Recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet. Parasitol. 2000, 93, 393–408.
- Paulsen, P.; Forejtek, P.; Hutarova, Z.; Vodnansky, M. Alaria alata mesocercariae in wild boar (Sus scrofa, Linnaeus, 1758) in south regions of the Czech Republic. Vet. Parasitol. 2013, 197, 384–387.
- Dollfus, R.P.; Chabaud, A.G. Distomum musculorum suis, mesocercairia of Alaria alata in the wild boar Sus scrofa L. Ann. Parasitol. Hum. Comp. 1953, 28, 354–364.
- Shimalov, V.; Shimalov, V. Helminth fauna of the American mink (Mustela vison Schreber, 1777) in Belorussian Polesie. Parasitol. Res. 2001, 87, 886–887.
- Renteria-Solis, Z.; Kolodziej-Sobocinska, M.; Riehn, K. Alaria spp. mesocercariae in Eurasian badger (Meles meles) and wild boar (Sus scrofa) from the Bialowieza Forest, north-eastern Poland. Parasitol. Res. 2018, 117, 1297–1299.
- Shimalov, V.V.; Shimalov, V.T. Helminth fauna of the wolf (Canis lupus Linnaeus, 1758) in Belorussian Polesie. Parasitol. Res. 2000, 86, 163–164.
- Sailer, A.; Glawisching, W.; Irschik, I.; Lücker, E.; Riehn, K.; Paulsen, P. Findings of Alaria alata mesocercariae in wild boar in Austria: Current knowledge, identification of risk factors and discussion of risk management options. Wien. Tierärztliche Mon. 2012, 99, 346–352.
- Gérard, C.; Franssen, F.; La Carbona, S.; Monteiro, S.; Cozma-Petrut, A.; Utaaker, K.S.; Jambrak, R.A.; Rowan, N.; Rodriguez-Lazaro, D.; Nasser, A.; et al. Inactivation of parasite transmission stages: Efficacy of treatments on foods of non-animal origin. Food Sci. Technol. 2019, 91, 12–23.
- González-Fuentes, H.; Hamedy, A.; Koethe, M.; von Borell, E.; Luecker, E.; Riehn, K. Effect of temperature on the survival of Alaria alata mesocercariae. Parasitol. Res. 2015, 114, 1179–1187.
- French Agency for Food, Environmental and Occupational Health and Safety. Opinion on the Presence of Mesocercarial Parasites of the Trematode Alaria alata in Wild Boar Meat. 2015. Available online: https://www.anses.fr/en/system/files/BIORISK2015SA0052EN.pdf (accessed on 12 July 2021).
- Fernandes, B.J.; Cooper, J.D.; Cullen, J.B.; Freeman, R.S.; Ritchie, A.C.; Scott, A.A.; Stuart, P.F. Systemic infection with Alaria americana (Trematoda). Can. Med Assoc. J. 1976, 115, 1111–1114.
- Kramer, M.H.; Eberhard, M.L.; Blankenberg, T.A. Respiratory symptoms and subcutaneous granuloma caused by mesocercariae: A case report. Am. J. Trop Med. Hyg. 1996, 55, 447–448.
- Beaver, P.C.; Little, M.D.; Tucker, C.F.; Reed, R.J. Mesocercaria in the Skin of Man in Louisiana. Am. J. Trop Med. Hyg. 1977, 26, 422–426.
- Shea, M.; Maberley, A.L.; Walters, J.; Freeman, R.S.; Fallis, A.M. Intraretinal larval trematode. Am. J. Ophthalmol. 1973, 77, 784–791.
- German Federal Institute for Risk Assessment (BfR). Game Meat: Health Assessment of Human—Pathogenic Parasites. Opinion No.045/2018, 1st ed.; German Federal Institute for Risk Assessment: Berlin, Germany, 2018.
- Portier, J.; Jouet, D.; Ferte, H.; Gibout, O.; Boireau, P.; Vallee, I. New Data in France on the Trematode Alaria alata (Goeze, 1792) Obtained during Trichinella Inspections. Parasite 2011, 18, 271–275.
- McDonald, H.R.; Kazacos, K.R.; Schatz, H.; Johnson, R.N. Two cases of intraocular infection with Alaria mesocercaria (Trematoda). Am. J. Ophthalmol. 1994, 117, 447–455.
- Gavrilovic, P.; Pavlovic, I.; Todorovic, I. Alaria alata mesocercariae in domestic pigs and wild boars in South Banat, northern Serbia. Comp. Immunol. Microbiol. Infect. Dis. 2019, 63, 142–144.
- González-Fuentes, H.; Hamedy, A.; von Borell, E.; Luecker, E.; Riehn, K. Tenacity of Alaria alata mesocercariae in home-made German meat products. Int. J. Food Microbiol. 2014, 176, 9–14.
- Krostitz, W. Der Wildfleischmarkt. Fleischwirtschaft 1996, 76, 7.
- Haldimann, M.; Baumgartner, A.; Zimmerli, B. Intake of lead from game meat—Arisk to consumers’ health? Eur. Food Res. Technol. 2002, 215, 375–379.
- Hoffman, L.C.; Wiklund, E. Game and venison—Meat for the modern consumer. Meat Sci. 2006, 74, 197–208.
