Electrophoretic deposition (EPD) is a powerful technique to assemble metals, polymer, ceramics, and composite materials into 2D, 3D, and intricately shaped implants. Polymers, proteins, and peptides can be deposited via EPD at room temperature without affecting their chemical structures. Furthermore, EPD is being used to deposit multifunctional coatings (i.e., bioactive, antibacterial, and biocompatible coatings).
- antibacterial
- bioactive
- bioactive glasses
- chitosan
- PEEK
- zein
- gelatin
- electrophoretic deposition
1. Introduction
EPD is a commonly applied method in the field of biomaterials, especially for producing bioactive coatings [1][2][3][4][5][6][8,9,10,11,12,13]. EPD is helpful in processing micro- and nanostructured biomaterials in simple as well as complex form [7][8][9][10][14,15,16,17]. EPD provides a simple setup consisting of two electrodes. Two kinds of power sources, alternating current (AC) and direct current (DC), can be applied for EPD [11][12][18,19]. The freedom to manipulate nanoparticles in the suspension has brought a lot of new interest to the process. The adjustable shape of the substrates, deposition of materials at room temperature, and the possibility to adjust the thickness as well as the morphology of the coatings by optimizing process parameters, such as applied voltage, the concentration of suspension, and deposition time, make EPD a potential processing method for depositing biopolymers for research purposes and also at large scale [13][14][20,21].
In the biomedical field, deposition of thin films or coatings on porous structures, such as on scaffolds, is of great interest, and has become an essential part of tissue engineering and regenerative medicine [15][16][17][18][19][22,23,24,25,26]. EPD allows a homogeneous coating consisting of bioactive layers and biological entities (proteins, polymers, enzymes, cells, etc.) with therapeutic metallic ions or drugs on various substrates [20][21][27,28]. The development of hydroxyapatite (HA) coatings on Ti substrate, confirming the induced bioactivity of the substrate, gained a lot of attention and initiated further investigations in 1986 [22][29].
Biomaterial coatings should possess some important properties for their effective utilization in the implant devices, such as biocompatibility, bioactivity, antibacterial effect, surface wettability, adhesion strength, corrosion, wear resistance, and so on. The interaction of coating surface and cells is of crucial consideration when designing the coating material. In some cases, there is a need to modify the surface properties of the base material by depositing an appropriate coating. For example, graphene oxide (GO) was applied on Al substrate to enhance the anticorrosive properties of Al [23][30]. Similarly, sometimes the surfaces are not responsive to the environment of their application, and we know that in case of biomaterials, wettability is an important factor. Again, EPD can alter the wetting behavior of the base materials [24][31]. The biomedical coatings are characterized on the basis of various investigative methods for the above-mentioned properties, for example, the formation of HA on coated samples in simulated body fluid (SBF) or phosphate buffer saline (PBS) solution for bioactivity, using cell-line studies for biocompatibility and cell attachment and growth, minimum inhibition zone tests for antibacterial effect, water contact angle measurement for wettability, pencil hardness and scratch tests for adhesion strength, and so on. The characterization of biomaterial coatings are further discussed in detail in the sections of chitosan, zein, and PEEK EPD coatings [25][26][27][28][32,33,34,35].
2. Electrophoretic Deposition
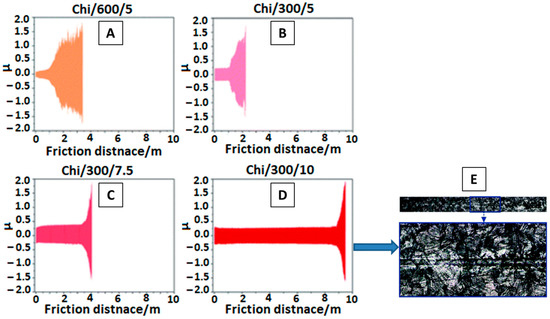
To assess the wear mechanism of chi coating on Ti15Mo alloy surface, Szklarska et al. [29][82] conducted tribological analysis. Coatings were deposited at different voltages for different time periods, as shown in Figure 12 A–D. The coating deposited at a high voltage of 10 V and for 300 s gave the best results for the abrasion test. The results were recorded in the form of a friction co-efficient (µ) vs. friction distance (m) plot. The wear track monitored by SEM for best coating deposited at 10 V for 300 s (Chi/300/10) is shown in Figure 12 E.
The above discussion gives a general idea of how chi is being employed in the biomedical field and how it is benefiting society. The main purpose in analyzing biopolymers like chi is to impart beneficial properties (bioactive and antibacterial) to such materials in metallic implants. As EPD is a facile and cost-effective method, and it can be tuned easily by adjusting suspension and process parameters to obtain desired coatings. The recent trend of co-substitution of bioceramics and metallic ion doping has greatly improved the bioactivity and antimicrobial effect of chitosan composite coatings. Still, there are challenges we need to face in terms of mechanical aspects and application of these composite coatings in real-life conditions.
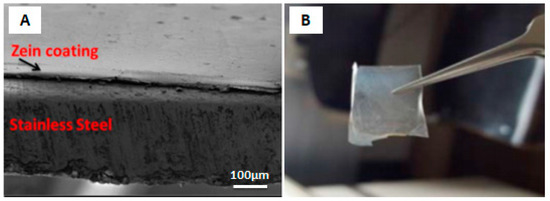
The mechanism behind the EPD of zein coatings was first investigated by Kaya et al. [30][84] at various voltages and deposition times on 316L-SS substrate. The EPD mechanism suggested in this work was based on the chemical reactions occurring in acidic environment during deposition. Smooth and homogeneous coatings were obtained, with the highest deposition yield at 10 V for 10 min. The coating was detached from the substrate to infer the self-standing status of the zein coating. The SEM micrograph showing film like coating (A) and digital image of detached film (B) is shown in the Figure 215 . As shown, the zein film had no scratch or crack after detaching from substrate.
In addition to adhesive strength and wear of the coatings, wear resistance of the coating is also important. It is important that the coatings should provide suitable wear resistance when in contact with the natural tissues. Poor wear resistance of the coatings leads to the release of wear debris in the body, which is often very painful for the patients. The combination of PEEK with various ceramic materials is being studied in the literature. It has been reported that PEEK-based composite coatings can improve the wear resistance of the coatings [31][32][33][34][89,97,107,165]. For example, Virk et al. [33][107] showed that PEEK/BG composite coatings showed better wear resistance. More interestingly, it was shown that the incorporation of h-BN in the PEEK/BG matrix further improves wear resistance. Furthermore, PEEK also provided the lubrication effect required in the orthopedic joints, which further confirms the suitability of PEEK and PEEK-based composites for biomedical applications.
3. Conclusions
The role of EPD in the polymer coating of chitosan, zein, PEEK, and their respective composite coatings with ceramics (HA, BG, GO, etc.) and metallic ions ( Cu, Ag, Sr, Mn, etc.) have been discussed in light of the available literature. The in vitro and in vivo response of these polymer and composite coatings related to biocompatibility, bioactivity, and antibacterial effect, along with their mechanical properties, are addressed in this review. Here, we summarize the conclusive comments and future perspectives for EPD of polymer composite coatings.
EPD is a facile and economical coating technique to process a variety of suspension combinations on a variety of simple and complex substrates. EPD is environmentally friendly. It also provides the ease of manufacturing and high control over the quality of the final product. A deep understanding of the EPD process kinetics and working conditions leads to the efficient fabrication of the intended product.
The biopolymers have good biocompatibility, and they impart bioactiveness to various substrate materials—for example, Ti, which is a biocompatible material, but not bioactive. The polymeric coating on Ti substrate enhances its bioactivity as an implant material, as discussed in Section 2.4 of this review. In light of above discussion, it is evident that not only do the biopolymers render a surface bioactive, but they can also control the degradation rate of a substrate (i.e., in the case of Mg substrate, discussed in Section 2.5 .
The challenges still facing the EPD of biopolymers are mostly related to the mechanical aspects of the coatings. Although the incorporation of various metallic ions and ceramics like HA, BG, silicates, and carbon-based materials (CNTs, GO) improve the wear and corrosion resistance of coatings and the recent trend of co-ion doping in MBGNs have augmented the behavior of coatings in real physiological conditions. Still, the adhesion and quality of coatings are not up to the mark, and a lot of research needs to be carried out to optimize the concentration of various elements in the coatings and EPD processing parameters to obtain high-quality coatings for orthopedic implants.
