Liquid biopsy, which surmounts the limitation of tissue biopsy, is evaluated as a potential tool for early cancer detection and monitoring. By sampling from blood, stool, urine, saliva, and other fluid samples, liquid biopsy provides a non-invasive and feasible cancer detection service. Compared with tissue biopsy, liquid biopsy is also more comprehensive to evaluate tumor heterogeneity since tumor sites can release aberrant signals into body fluid.
- machine learning
- early cancer detection
- liquid biopsy
1. Introduction
When cells mutate, they could divide uncontrollably and eventually form cancer [1]. According to the World Health Organization, cancer accounts for nearly 10 million deaths in 2020. Unfortunately, this number is estimated to be still climbing in the following decades and will reach 27 million new cases in 2040 [2]. As the second factor of death, cancer accounts for one-sixth of deaths worldwide each year [3]. Therefore, fighting against cancer is a huge challenge for global public health. Early detection, followed by tailored site-specific treatment, plays an important role in the front-line cure of cancer and could reduce the eventual mortality of cancer patients [4][5][6][4,5,6].
Cancer is associated with mutated genes; and genetic analysis is increasingly applied in cancer diagnosis [7]. The traditional methods for genetic testing on cancer patients are sampling from tumor tissues. However, tumor tissue biopsy is limited by several drawbacks such as invasive acquisition, clinical complications, sample preservation, and tumor heterogeneity [8][9][10][8,9,10].
Liquid biopsy [7][11][7,11], which surmounts the limitation of tissue biopsy, is evaluated as a potential tool for early cancer detection and monitoring [12]. By sampling from blood, stool, urine, saliva, and other fluid samples, liquid biopsy provides a non-invasive and feasible cancer detection service [13][14][15][16][13,14,15,16]. Compared with tissue biopsy, liquid biopsy is also more comprehensive to evaluate tumor heterogeneity since tumor sites can release aberrant signals into body fluid [17][18][17,18]. Researchers paid significant attention to the different components from liquid biopsy which are associated with cancers [19][20][21][22][23][19,20,21,22,23].
As the possibility or severity of tumor in the body is relevant to the liquid biopsy components, accurate cancer prediction based on the characteristics of these components becomes a significant problem. The application of machine learning protocols has been widely studied in recent years, proving to be valuable in early cancer detection. Nevertheless, the required knowledge to implement these methods is high, posing an obstacle to researchers who are looking to get started on liquid biopsy analysis and early cancer detection.
2. Liquid Biopsy Components
During the formation and growth of primary tumors, cells undergo active release, necrosis, or apoptosis [24][25][99,100]. In these process, various components are released into the liquid, including circulating tumor cells, cell-free DNA, circulating tumor DNA, cell-free RNA, exosomes, and tumor educated platelets(TEPs) [26][101].
The presence of circulating Tumor Cells (CTCs) was firstly identified by Ashworth (Australia) in 1869 [27][102]. When Ashworth performed an autopsy on a metastatic breast cancer patient, cells similar to those from the primary tumor were found in the blood. CTCs are currently defined as the tumor cells that shed or migrate actively into the vessel from the primary tumor or metastatic sites and then circulate in the bloodstream [28][103]. The opinion of tumor self-seeding suggests that CTCs can recirculate back, resulting in the possibility of metastases, which is responsible for the majority of deaths associated with cancer [29][30][104,105]. As the access to peripheral blood circulation is a prerequisite for distant metastasis of tumors [31][106], detection of tumor cells in blood will indicate the possibility of distant metastasis of tumors [32][107].
CTC is isolated from peripheral blood, which can avoid invasive and complex biopsy procedures. The culture of tumor cell lines takes a long time and is homogeneous, which cannot accurately reflect the genetic diversity and the changing tumor microenvironment. In contrast, CTCs-derived xenografts can reflect the biological characteristics of cancer more accurately, providing a visual window for studying the dynamic evolution of cancer and allowing monitoring of the longitudinal evolution of tumors at the molecular level.
Platelets (also termed thrombocytes) are the second most abundant cell types in peripheral blood, existing as circulating anucleated cell fragments. The largest platelets are about 2–3 microns in diameter [33][139]. More recently, platelets are implicated a central role in the local and systemic responses to tumor growth [34][35][140,141]. Confrontation of platelets with tumor cells by transferring tumor-associated biomolecules (‘education’) is an emerging research field resulting in the term of tumor-educated platelets (TEPs).
3. Machine Learning Algorithms and Clinical Application in Early Cancer Detection Based on Liquid Biopsy
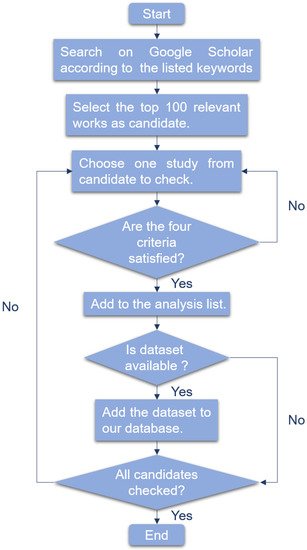
-
The research is about liquid biopsy.
-
The research is about cancer detection.
-
The research utilized corresponding machine learning method.
-
For several models compared, we only consider the model which performs best.
