Viral vectors and viral vaccines are invaluable tools in prevention and treatment of diseases. About 14% of vaccines approved by the FDA involve enveloped viruses, while out of the 15 gene therapy products approved worldwide in 2019, six of them use enveloped viruses, and 39% of gene therapy clinical trials are using enveloped viruses. Enveloped viruses are encased in a lipid bilayer which, in most cases, fuses with the target host cell membrane to infect cells. These enveloped viruses are produced in various systems, including traditional embryonated chicken eggs or more advanced cell culture technologies such as MRC-5 cells, Vero cells and HEK293-derived cell lines. The manufacturing of viral vector and viral vaccine products has always been paved with challenges related to the downstream processing. Purification process unit operations usually start with harvest and clarification, followed by intermediate purification steps, before polishing and formulation steps. Although techniques have greatly improved over the years to generate purer high-quality products and reproducible processes while maintaining or decreasing the cost of goods, regulatory agencies are increasingly stringent regarding product identity and characterization of the end products and level of acceptable impurities as a way to ensure public safety and maintain public trust in this class of medicine.
- extracellular vesicles
- enveloped viruses
- lentiviral vectors
- viral vaccines
- purification process
1. Viral Purification Processes
No unique stream exists in the downstream processing of viral vectors and vaccines. Indeed, not only does each virus have its own properties and behavior, but the treatment that viruses can undergo also depends on the nature of the final product: Should the virus be inactivated or live-attenuated retaining infectivity properties, does the particle structure have to be maintained for immunogenicity, or should the virus, that might be defective, retain the properties to effectively transduce cells and express the targeted transgene, as is the case for viral vectors used in gene therapy or vaccination? Traditional techniques for purifying EVs and viruses involved ultracentrifugation and filtration and are still being used extensively. However, more advanced chromatographic steps and scalable technologies are being implemented. Common steps and process unit operations are presented in a generic sequence summarizing the purification strategy (
). In the case of enveloped viruses, lysis of the cells is not required as the viruses bud out of the cell membranes. Therefore, the first clarification step aims at removing cells and cell debris. Centrifugation and filtration are the most commonly used techniques at this stage. In general, one or multiple purification steps follow in order to concentrate the virus and remove host cell proteins (HCPs) and host cell DNA. They might include tangential flow filtration and chromatographic unit operations.
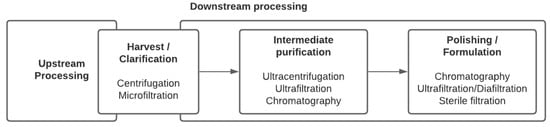
General sequence of viral vector and viral vaccine production bioprocesses.
2. Harvest and Clarification
In both cell culture or egg production systems, the transition step between upstream and downstream processing is known as the harvest. As stated, in the case of enveloped viruses or EVs, since particles directly bud out of the cells, there is no need to use detergents to lyse the cells. Therefore, downstream processing starts directly with removing cells and large debris. It is often completed in two steps, combining centrifugation and filtration.
2.1. Centrifugation
Centrifugation is a common way to remove cells and large cellular debris by pelleting them. It is still used broadly despite the high cost and difficulty to scale up as it offers good recovery [1]. Based on their lower density, viruses and EVs are therefore both recovered in the supernatant during this step.
2.2. Microfiltration
Microfiltration is referred to using filters with membrane cut-offs usually between 0.1 and 10 µm. Different filtration techniques are used with such filters, the main ones being normal-flow filtration (NFF) and tangential flow filtration (TFF). TFF, according to its name, differs from NFF in the flow directionality. Both have been extensively and very efficiently used in the separation and purification of biotherapeutics.
Different types of filters can be used in NFF: dead-end filters and depth filters. Dead-end filters have defined pore sizes, and excluded particles are retained only at the surface, whereas depth filters are made of porous material, which can retain particles of different sizes across the membrane’s thickness [2], rendering membrane fouling less problematic. Depth filters can also be positively charged to effectively capture host cell DNA and HCPs. Both types of filters have the advantage of being easy to scale up and cost-effectiveness. A scalable process using a dual 0.45–0.2 µm filter has been proposed to clarify retroviral vectors produced in HEK293-derived cells [3]. NFF has also been used effectively for decades in the clarification process of influenza virus produced in embryonated chicken eggs [4].
In TFF, biological fluids recirculate in parallel to the membrane surface, preventing cake formation. Particles smaller than the pore size flow through the membrane in the permeate, while larger particles are retained by the membrane and are recovered in the retentate. TFF is also a highly scalable method and has been successfully implemented in the manufacturing process of smallpox and monkeypox vaccine JYNNEOS [5].
Given that these filters separate particles mainly based on their size and given the overlapping size range of viruses and EVs, both types of particles remain in the filtrate or permeate during this step.
3. Concentration and Intermediate Purification
3.1. Traditional Ultracentrifugation
Traditional techniques for virus separations were based on their physical characteristics such as their size and density. Ultracentrifugation is a well-established technique that has been used for decades to pellet low-density particles. It can be used in one step, stepwise (differential (ultra-) centrifugation), with continuous density gradients or discontinuous density gradients called cushions with media such as cesium chloride, iodixanol or sucrose.
In the field of gene therapy, lentiviral vectors have been concentrated and partially purified using ultracentrifugation [6]. Iodixanol gradients [7] or sucrose cushions [8] have been used for purifying lentivirus preparations. Ultracentrifugation steps using sucrose gradients have also been used to purify retrovirus [9].
In the field of vaccines, ultracentrifugation and zonal-rate separation on sucrose cushions has been used widely for the purification of influenza virus. The FluMist vaccine, for example, employs ultracentrifugation in the production process. Japanese Encephalitis virus for the preparation of Ixiaro vaccine is purified using sucrose density gradients [10].
In the field of EVs, differential ultracentrifugation was for a while the gold standard to isolate EVs [11], with sequential steps of increased centrifugal force. It is no longer the method of choice, however, as it is cumbersome, induces higher variability than other techniques and has been shown to damage and aggregate EVs [12]. Ultracentrifugation with sucrose or iodixanol gradients is another popular approach to isolating EVs [13].
Ultracentrifugation using continuous density gradients is limited by the volume that can be processed (usually less than 50 mL); it is therefore mostly used for pre-clinical material or small-scale research samples. Continuous-flow centrifugation overcomes this volume limitation and is still being used at a large scale in vaccine manufacturing, especially in the case of influenza vaccine and Japanese encephalitis vaccine. However, it does not translate well for lentiviral vectors, which are subject to a loss of infectivity by ultracentrifugation with or without sucrose gradients [14]. Continuous-flow centrifugation equipment is also high-maintenance, costly and voluminous. Moreover, due to the overlapping density of EVs and viruses, effective separation cannot be achieved. Ultracentrifugation is therefore not a suitable method to separate enveloped viruses from EVs, and viral manufacturing processes that rely on ultracentrifugation in downstream processing should expect retention of EVs in the bulk product if an additional step segregating the two entities is not considered.
3.2. Ultrafiltration Tangential Flow Filtration
Ultrafiltration (UF) is another membrane separation technique with tighter pore sizes than in microfiltration, usually ranging from 1 to 100 nm. It is most commonly used in TFF mode to concentrate the products of interest, and combined with diafiltration (DF), it allows buffer exchange. This well-controlled and scalable technology induces very low shear stress, which makes it very popular in various biomanufacturing processes. UF/DF is widely used in the field of influenza virus production [15] using different membrane molecular weight cut-offs (MWCO), from 100 to 750 kDa. UF/DF is also a method of choice in the purification of retroviral and lentiviral vectors using 100 to 300 kDa membranes [16][17]. UF/DF has also been employed in the field of EVs, especially for its scalability advantage [18][19].
In UF/DF TFF, most viruses and EVs are larger than the molecular weight cut-off of the membrane. As they have very similar size ranges, as in any filtration technique, they are both recovered in the same phase, here in the retentate, while smaller particles pass through the permeate. Therefore, TFF cannot be used for separating EVs from enveloped viruses.
3.3. Chromatography
Chromatography is a commonly used process unit in the downstream processing of viral vectors and viral vaccines. Its role is to capture the particle of interest (bind-elute mode) or impurities (flow-through mode). If the virus is bound to the chromatographic material, it is then eluted, allowing its purification and concentration. Separation by chromatography is based on the physicochemical interactions of the particles of interest with the solid phase in opposition to the contaminants or impurities.
Different supports exist for the solid phase, also called stationary phase.
The most traditional one is resin-based chromatography, using packed-bed columns of microbeads with specific chemical properties. Packed-bed chromatography is, however, mainly used in small molecule purification such as antibodies, as the larger size of viruses affects their diffusion into the pores of the adsorbent resin thus reducing the dynamic binding capacity.
Alternative chromatographic supports are convective media such as membrane adsorbers and monoliths through which processing time, capacity and recovery are improved for viral processes rendering them more cost effective. Membrane adsorbers are a combination of liquid chromatography and membrane filtration [20]. They offer reduced diffusion times compared to packed-bed chromatography, with high flow rate operation capabilities and low pressure drops. The low dead volume of the system also yields reduced buffer consumption. Although reusability is always an option, another advantage of membrane adsorbers resides in their suggested single-use format, removing the need for lengthy validated clean-in-place and regeneration procedures and eliminating the risk of cross-contamination. Membrane adsorbers have been successfully used for scalable processes of lentiviral and retroviral vectors with high titers [21].
The disposability advantage also goes for monoliths. Monoliths, also known as convective interaction media (CIM) are made of porous materials organized in a single block with highly cross-linked macropores with a diameter range of 10 to 4000 nm [22]. Similar to membrane chromatography, mass transport is essentially convective, allowing high flow rates and low pressure drops. Most chromatographic monoliths are made of polymethacrylate material and are operated at a large scale with radial flow devices. Monoliths have shown great performance in the purification of influenza virus and lentiviral vectors as compared to other chromatographic means [23]. Rubella virus is another example of an enveloped viral vaccine and has been efficiently concentrated and purified using a monolith with almost 100% recovery and maintained infectivity [24].
Chromatographic materials can also be characterized by surface chemistry. Ion-exchange, hydrophobic interaction, affinity, size-exclusion and mixed-mode are the main types.
Ion-exchange chromatography (IEX) is the most commonly used technique and is based on difference in charge between the viral envelope and the stationary phase. It is mostly operated in bind-elute mode. IEX usually offers high resolution especially when using elution gradients to fractionate closely related biomolecules. Depending on the particle of interest’s net charge, either anion or cation exchange is employed. Most viruses are negatively charged at physiological pH due to their isoelectric point (pI) being below 7.4. Interestingly, egg-derived influenza virus has been purified by both anion-exchange (AIEX) and cation-exchange (CIEX) chromatography, although AIEX was more favorable [15]. Lentiviral vectors, as well as retroviral vectors have been purified at a large scale using AEX, yielding 22% to over 60% of recovery of infectious particles [6][16].
The use of hydrophobic interaction chromatography (HIC) is scarcer. It is mainly known for the purification of vaccinia virus [25]. The reason behind the low popularity of the method is due to the high salt concentration used for desorption, which can be detrimental to the virus integrity and functionality, especially in the case of viral vectors used in gene therapy.
Affinity chromatography (AC) separation is based on specific interactions between the particles of interest and the stationary phase and is used in bind-elute mode. It has attracted interest in recent years. The advantage of AC is the high specificity of the interaction, yielding highly pure product in one step. Mechanisms of affinity include specific antigen–antibody interactions, which, when employed for the purification of measles virus [26], outperformed ultracentrifugation. Mumps virus purification using AC with a monolithic column coupled with polyclonal antibodies is another example [27]. Lectin affinity chromatography uses lectin ligands binding to specific carbohydrates via carbohydrate recognition domains. It was used in the purification of influenza A virus [28] and in the purification of HSV-1 [29]. Immobilized metal affinity chromatography (IMAC) is based on metal ion affinity such as zinc, cobalt, nickel or a combination of copper, cobalt and nickel and is used for the purification of influenza virus [30], HSV-1 [31], retroviral vectors [32] and lentiviral vectors [33], respectively. An additional example of AC mechanism is based on heparin affinity and has been very popular for the purification of many enveloped viruses, including HSV-1, vaccinia Ankara virus and retroviral and lentiviral vectors [34]. Despite the great performance of AC, it is not often implemented at a large scale due to the high cost of ligand design and immobilization.
Mixed-mode chromatography (MMC) is based on the combination of various multimodal binding mechanisms, such as ligands combining ionic interaction, hydrophobic interaction and hydrogen bonding. Hydroxyapatite, a complex crystalline compound, which resin binds at the same time negatively charged phosphate groups and positively charged functional groups, is a good example of MMC. It has shown recovery of up to 46% in the purification of retroviral vectors [35].
In the field of EVs, the use of AIEX has also been reported to efficiently isolate EVs from HEK293T cell cultures [36]. No studies attempting to separate viruses from coproduced EVs have been reported thus far. Despite the sizeable challenge, as charges between intermediate entities can exhibit slight differences, the possibility thus remains that this technique could separate EVs from viruses, as a recent study in another context showed the feasibility of separating full and empty adeno-associated virus (AAV) capsids [37].
When approaching AC techniques in the field of EVs, immunoaffinity appears appealing and has been widely employed in the isolation of EVs from cell culture or body fluids [38]. Tetraspanin proteins found at the surface of EVs are often reported as target molecules. However, specificity has not been demonstrated for efficient separation of EVs from viruses. Indeed, tetraspanins were also associated with viruses, such as CD63 with retroviral vectors and CD81 with lentiviral vectors [18][39]. Distinctive markers have yet to be accurately identified, and they may differ depending on the expression system and the produced enveloped virus. As documented in previous studies, EVs coproduced with enveloped viruses carry similar membrane proteins, and more extensive studies would be needed to identify and validate any specific markers that would enable separation of these two entities.
4. Polishing
Polishing is one of the last steps in bioprocessing, allowing the removal of remaining impurities, and can be completed after the final formulation of the product. This step is critical as it should ensure the purity, quality and potency of the final product according to stringent regulatory requirements.
Size exclusion chromatography (SEC) is the most commonly used chromatographic technique, based on the molecular size difference between the particle of interest and the impurities. SEC is used for example in the late-stage purification of lentiviral vectors [40]. Although still broadly used, SEC induces dilution of the final product and has usually low capacity.
Another MMC example used for polishing is the combination of size exclusion and binding properties of the Capto™ Core 700 and 400 resins. These are used in flow-through mode as the particles of interest are recovered in the flow-through, while impurities bind to the high-capacity column. It was originally designed for the removal of ovalbumin in the purification of influenza virus produced in eggs [41] but has since been applied to other viruses such as lentiviral vectors [40].
SEC and more recently Capto™ Core have been successfully implemented in the isolation processes of EVs [19][38][42].
Both are well-controlled technologies and scalable; however, since their separation principle is based on size, they cannot efficiently separate EVs from viruses due to their size similarities.
References
- Besnard, L.; Fabre, V.; Fettig, M.; Gousseinov, E.; Kawakami, Y.; Laroudie, N.; Scanlan, C.; Pattnaik, P. Clarification of vaccines: An overview of filter based technology trends and best practices. Biotechnol. Adv. 2016, 34, 1–13.
- Schmidt, S.R.; Wieschalka, S.; Wagner, R. Single-Use Depth Filters: Application in Clarifying Industrial Cell Cultures—BioProcess International. BioProcess Int. 2017, 16, 6–11.
- Segura, M.M.; Kamen, A.; Trudel, P.; Garnier, A. A novel purification strategy for retrovirus gene therapy vectors using heparin affinity chromatography. Biotechnol. Bioeng. 2005, 90, 391–404.
- Goyal, S.M.; Hanssen, H.; Gerba, C.P. Simple method for the concentration of influenza virus from allantoic fluid on microporous filters. Appl. Environ. Microbiol. 1980, 39, 500.
- U.S. Food & Drug Administration. Package Insert—JYNNEOS; Bavarian Nordic A/S: Kvistgaard, Denmark, 2019.
- Moreira, A.S.; Cavaco, D.G.; Faria, T.Q.; Alves, P.M.; Carrondo, M.J.T.; Peixoto, C. Advances in Lentivirus Purification. Biotechnol. J. 2021, 16, e2000019.
- Kishishita, N.; Takeda, N.; Anuegoonpipat, A.; Anantapreecha, S. Development of a pseudotyped-lentiviral-vector-based neutralization assay for chikungunya virus infection. J. Clin. Microbiol. 2013, 51, 1389–1395.
- Olgun, H.B.; Tasyurek, H.M.; Sanlioglu, A.D.; Sanlioglu, S. High-Grade Purification of Third-Generation HIV-Based Lentiviral Vectors by Anion Exchange Chromatography for Experimental Gene and Stem Cell Therapy Applications. In Skin Stem Cells: Methods and Protocols; Turksen, K., Ed.; Springer: New York, NY, USA, 2019; pp. 347–365.
- Rodrigues, T.; Carrondo, M.J.T.; Alves, P.M.; Cruz, P.E. Purification of retroviral vectors for clinical application: Biological implications and technological challenges. J. Biotechnol. 2007, 127, 520–541.
- U.S. Food & Drug Administration. Package Insert and Patient Information—IXIARO; Valneva Austria GmbH: Vienna, Austria, 2018.
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22.
- Mol, E.A.; Goumans, M.-J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2061–2065.
- Van Deun, J.; Mestdagh, P.; Sormunen, R.; Cocquyt, V.; Vermaelen, K.; Vandesompele, J.; Bracke, M.; De Wever, O.; Hendrix, A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles 2014, 3.
- Perry, C.; Rayat, A. Lentiviral Vector Bioprocessing. Viruses 2021, 13, 268.
- Wolff, M.W.; Reichl, U. Downstream Processing: From Egg to Cell Culture-Derived Influenza Virus Particles. Chem. Eng. Technol. 2008, 31, 846–857.
- Rodrigues, T.; Carvalho, A.; Carmo, M.; Carrondo, M.J.T.; Alves, P.M.; Cruz, P.E. Scaleable purification process for gene therapy retroviral vectors. J. Gene Med. 2007, 9, 233–243.
- Geraerts, M.; Michiels, M.; Baekelandt, V.; Debyser, Z.; Gijsbers, R. Upscaling of lentiviral vector production by tangential flow filtration. J. Gene Med. 2005, 7, 1299–1310.
- Do Minh, A.; Star, A.T.; Stupak, J.; Fulton, K.M.; Haqqani, A.S.; Gélinas, J.-F.; Li, J.; Twine, S.M.; Kamen, A.A. Characterization of Extracellular Vesicles Secreted in Lentiviral Producing HEK293SF Cell Cultures. Viruses 2021, 13, 797.
- Lobb, R.J.; Becker, M.; Wen Wen, S.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031.
- Orr, V.; Zhong, L.; Moo-Young, M.; Chou, C.P. Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 2013, 31, 450–465.
- Zimmermann, K.; Scheibe, O.; Kocourek, A.; Muelich, J.; Jurkiewicz, E.; Pfeifer, A. Highly efficient concentration of lenti- and retroviral vector preparations by membrane adsorbers and ultrafiltration. BMC Biotechnol. 2011, 11, 55.
- Lynch, K.B.; Ren, J.; Beckner, M.A.; He, C.; Liu, S. Monolith columns for liquid chromatographic separations of intact proteins: A review of recent advances and applications. Anal. Chim. Acta 2019, 1046, 48–68.
- Kramberger, P.; Urbas, L.; Štrancar, A. Downstream processing and chromatography based analytical methods for production of vaccines, gene therapy vectors, and bacteriophages. Hum. Vaccines Immunother. 2015, 11, 1010–1021.
- Forcic, D.; Brgles, M.; Ivancic-Jelecki, J.; Santak, M.; Halassy, B.; Barut, M.; Jug, R.; Markušić, M.; Strancar, A. Concentration and purification of rubella virus using monolithic chromatographic support. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 981–986.
- Hansen, S.P.; Rene, F.; Udo, R.; Michael, W.; Gram, A.P. Purification of Vaccinia Viruses Using Hydrophobic Interaction Chromatography. U.S. Patent US20110306114A1, 23 August 2011.
- Njayou, M.; Quash, G. Purification of measles virus by affinity chromatography and by ultracentrifugation: A comparative study. J. Virol. Methods 1991, 32, 67–77.
- Brgles, M.; Sviben, D.; Forčić, D.; Halassy, B. Nonspecific native elution of proteins and mumps virus in immunoaffinity chromatography. J. Chromatogr. A 2016, 1447, 107–114.
- Opitz, L.; Salaklang, J.; Büttner, H.; Reichl, U.; Wolff, M.W. Lectin-affinity chromatography for downstream processing of MDCK cell culture derived human influenza A viruses. Vaccine 2007, 25, 939–947.
- Olofsson, S.; Jeansson, S.; Lycke, E. Unusual lectin-binding properties of a herpes simplex virus type 1-specific glycoprotein. J. Virol. 1981, 38, 564–570.
- Opitz, L.; Hohlweg, J.; Reichl, U.; Wolff, M.W. Purification of cell culture-derived influenza virus A/Puerto Rico/8/34 by membrane-based immobilized metal affinity chromatography. J. Virol. Methods 2009, 161, 312–316.
- Jiang, C.; Wechuck, J.B.; Goins, W.F.; Krisky, D.M.; Wolfe, D.; Ataai, M.M.; Glorioso, J.C. Immobilized Cobalt Affinity Chromatography Provides a Novel, Efficient Method for Herpes Simplex Virus Type 1 Gene Vector Purification. J. Virol. 2004, 78, 8994–9006.
- Ye, K.; Jin, S.; Ataai, M.M.; Schultz, J.S.; Ibeh, J. Tagging Retrovirus Vectors with a Metal Binding Peptide and One-Step Purification by Immobilized Metal Affinity Chromatography. J. Virol. 2004, 78, 9820–9827.
- Cheeks, M.C.; Kamal, N.; Sorrell, A.; Darling, D.; Farzaneh, F.; Slater, N.K.H. Immobilized metal affinity chromatography of histidine-tagged lentiviral vectors using monolithic adsorbents. J. Chromatogr. A 2009, 1216, 2705–2711.
- Zhao, M.; Vandersluis, M.; Stout, J.; Haupts, U.; Sanders, M.; Jacquemart, R. Affinity chromatography for vaccines manufacturing: Finally ready for prime time? Vaccine 2019, 37, 5491–5503.
- Kuiper, M.; Sanches, R.M.; Walford, J.A.; Slater, N.K. Purification of a functional gene therapy vector derived from Moloney murine leukaemia virus using membrane filtration and ceramic hydroxyapatite chromatography. Biotechnol. Bioeng. 2002, 80, 445–453.
- Heath, N.; Grant, L.; De Oliveira, T.M.; Rowlinson, R.; Osteikoetxea, X.; Dekker, N.; Overman, R. Rapid isolation and enrichment of extracellular vesicle preparations using anion exchange chromatography. Sci. Rep. 2018, 8, 5730.
- Joshi, P.R.H.; Bernier, A.; Moço, P.D.; Schrag, J.; Chahal, P.S.; Kamen, A. Development of a scalable and robust AEX method for enriched rAAV preparations in genome-containing VCs of serotypes 5, 6, 8, and 9. Mol. Methods Clin. Dev. 2021, 21, 341–356.
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 2021, 1636, 461773.
- Segura, M.M.; Garnier, A.; Di Falco, M.R.; Whissell, G.; Meneses-Acosta, A.; Arcand, N.; Kamen, A. Identification of host proteins associated with retroviral vector particles by proteomic analysis of highly purified vector preparations. J. Virol. 2008, 82, 1107–1117.
- Boudeffa, D.; Bertin, B.; Biek, A.; Mormin, M.; Leseigneur, F.; Galy, A.; Merten, O.W. Toward a Scalable Purification Protocol of GaLV-TR-Pseudotyped Lentiviral Vectors. Hum. Gene Ther. Methods 2019, 30, 153–171.
- Blom, H.; Åkerblom, A.; Kon, T.; Shaker, S.; van der Pol, L.; Lundgren, M. Efficient chromatographic reduction of ovalbumin for egg-based influenza virus purification. Vaccine 2014, 32, 3721–3724.
- Corso, G.; Mäger, I.; Lee, Y.; Görgens, A.; Bultema, J.; Giebel, B.; Wood, M.J.A.; Nordin, J.Z.; Andaloussi, S.E. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci. Rep. 2017, 7, 11561.
