1. Phenolic Compounds from Lignin Oxidation
The history behind utilizing lignin as a source of valuable phenolic compounds can be dated back to the mid-twentieth century as part of the paper industry’s search for new valorization pathways for lignin [1][2]. However, it is well known that the structural transformations and chemical treatments suffered by lignin have a considerable influence on the formation of phenolic compounds since modified lignin has less availability of phenolic precursors in its structure. Alkaline oxidation converts lignin into a complex mixture of products that could be phenolic monomers, dimers, and oligomers. The selectivity and efficiency of oxidative depolymerization depend strongly on processing conditions and lignin origin. Alkaline oxidation of softwood lignins produces mainly vanillin and vanillic acid, while syringaldehyde and syringic acid are obtained from hardwood lignins. A literature overview of some representative works about alkaline oxidations of lignin using oxygen shows values in the range of 4–12%
The history behind utilizing lignin as a source of valuable phenolic compounds can be dated back to the mid-twentieth century as part of the paper industry’s search for new valorization pathways for lignin [52,53]. However, it is well known that the structural transformations and chemical treatments suffered by lignin have a considerable influence on the formation of phenolic compounds since modified lignin has less availability of phenolic precursors in its structure. Alkaline oxidation converts lignin into a complex mixture of products that could be phenolic monomers, dimers, and oligomers. The selectivity and efficiency of oxidative depolymerization depend strongly on processing conditions and lignin origin. Alkaline oxidation of softwood lignins produces mainly vanillin and vanillic acid, while syringaldehyde and syringic acid are obtained from hardwood lignins. A literature overview of some representative works about alkaline oxidations of lignin using oxygen shows values in the range of 4–12% w
/
w
lignin for vanillin and 5–20%
w
/
w lignin for syringaldehyde, depending on the origin, type, and processing of each lignin [3][4][5][6][7][8][9][10].
lignin for syringaldehyde, depending on the origin, type, and processing of each lignin [8,22,39,43,44,48,49,54].
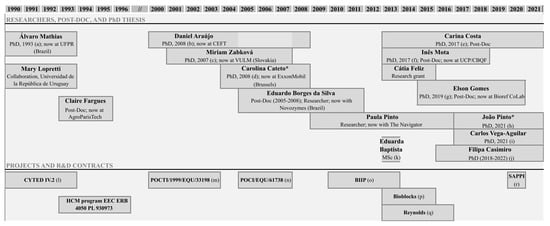
Lignin valorization is a solid research field, of more than 30 years, at the Laboratory of Separation and Reaction Engineering (LSRE) (
Figure 1). The LSRE group has vast experience in studying alkaline oxidation using oxygen to produce added-value phenolic compounds from lignin, namely vanillin and syringaldehyde. The potential of several lignins and liquors from different sources of biomass and delignification processes was evaluated through batch [11][5][12][6][7][8][13] and/or continuous experiments [14][15][16].
). The LSRE group has vast experience in studying alkaline oxidation using oxygen to produce added-value phenolic compounds from lignin, namely vanillin and syringaldehyde. The potential of several lignins and liquors from different sources of biomass and delignification processes was evaluated through batch [26,39,41,43,44,48,55] and/or continuous experiments [56,57,58].
Figure 1.
Research in lignin valorization started by Professor Alírio Rodrigues at LSRE (* thesis supervised by Prof. M. Filomena Barreiro (IPB, Portugal)). (
a
) Álvaro Luiz Mathias (UF Paraná, Brazil), “Production of vanillin from kraft lignin: Kinetics and processes”, 1993; (
b
) Daniel Araújo, “Production of vanillin from lignin present in the Kraft black liquor of the pulp and paper industry”, 2008; (
c
) Miriam Zabkova (Slovakia), “Clean technologies for the purification of wastewaters: adsorptive parametric pumping”, 2007; (
d
) Carolina Cateto, “Lignin-based polyurethanes: synthesis, characterization and applications”, 2008; (
e
) Carina Costa, “Vanillin and syringaldehyde from side streams of pulp & paper industries and biorefineries”, 2017; (
f
) Inês Mota, “Fractionation of syringaldehyde and vanillin from oxidation of lignin”, 2017; (
g
) Elson Gomes, “Development of a continuous process for the production of vanillin and syringaldehyde from kraft black liquor”, 2019; (
h
) João Pinto (IPB), “Development of sustainable polymer solutions”, 2019; (
i
) Carlos Alberto Vega-Aguilar (Costa Rica), “Dicarboxylic acids from lignin”, 2021; (
j
) Filipa Casimiro (IPB), “Studies on lignin oxidation and degradation of phenolic products”, 2022; (
k
) Eduarda Baptista, “Ultrafiltração de extrato de casca de Eucalyptus globulus para recuperação de compostos polifenólicos”, 2013; (
l
) CYTED IV.2—“Transformación de lignina en produtos de alto valor agregado”; (
m
) POCTI/1999/EQU/33198—“Development of an integrated process for the production of vanillin from the black liquor of the pulp industry”; (
n
) POCI/EQU/61738/2004—“Purification of vanillin from the lignin oxidation broth”, 2007; (
o
) BIIPP (SI IDT—11551/2010)—“Biorefinaria Integrada na Indústria da Pasta e Papel”; (
p
) BIOBLOCKS—“Concepção de produtos de base biológica como precursores para a bioindústria de síntese química e de biomateriais a partir de fontes lenhocelulósicas”; (
q
) FEUP/RJR/2014/01—Production of Vanillin from tobacco biomass lignin (R. J. Reynolds Tobacco Company, USA); (
r
) Collaboration with SAPPI—“Lignosulphonate’s characterization”, 2020.
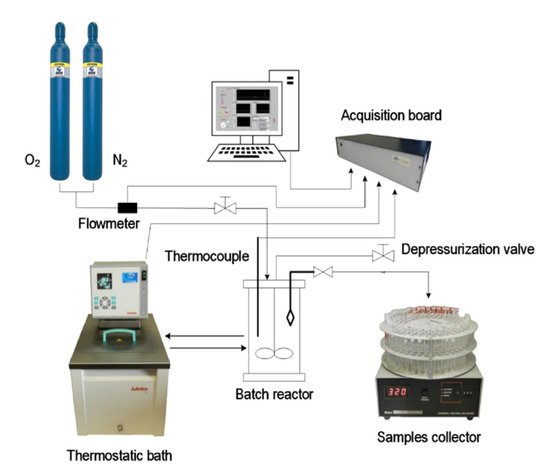
The batch oxidation experiments were performed in a jacketed reactor with a capacity of 1 L with initial temperature and pressure control at the beginning of the reaction. During the oxidation, the system’s total pressure was kept constant through the continuous feeding of oxygen to the reactor, and the reaction mixture (solution of lignin in NaOH with a selected concentration) was maintained under stirring. The experimental setup used for batch oxidation experiments at LSRE is presented in
Figure 2
.
Figure 2. Experimental setup used in batch oxidation experiments at LSRE. (Reprinted with permission from [17]. Copyright 2019 American Chemical Society.)
Experimental setup used in batch oxidation experiments at LSRE. (Reprinted with permission from [59]. Copyright 2019 American Chemical Society.)
The effect of one or several process parameters on lignin oxidative depolymerization performed through batch experiments has been intensively investigated with the primary objective of achieving the ideal conditions for obtaining the maximum conversion of lignin while avoiding the oxidation of the phenolic monomers produced [3][12][6][18][8][10][13][17]. The origin, composition, and processing of lignin (pulping process, isolation method, pretreatment, etc.), the oxygen partial pressure, the initial temperature, and the lignin and sodium hydroxide concentration in the reaction solution were studied, and its effect on the selectivity and efficiency of alkaline oxidative depolymerization process was evaluated [3][8][19][14][17][20]. It was found that a high partial pressure of oxygen reduces reaction time but leads to an increased rate of vanillin oxidation. Increasing the oxygen pressure accelerated both product formation and degradation, and therefore shortened the time needed to reach the maximum product yields [6][21]. In similar works, Schutyser and coworkers found that lignin oxidation under an inert atmosphere produced mainly oligomeric products, while the same reaction under oxygen primarily generated monomeric products [22]. Concerning the effect of pH of the mixture, it was concluded that during the lignin oxidation process, the yield of vanillin decreased when the pH value began to decrease. Moreover, there was a smaller vanillin degradation for strong alkaline conditions that increased significantly when pH was smaller than 11.5 [12][18]. Consequently, high alkali concentrations (pH > 12) are needed to reduce vanillin degradation. For temperature, it was found that an increase in this reaction condition can shorten the reaction time but, on the other hand, results in faster degradation of the aldehydes produced. However, Pacek and coworkers verified that even if usual reaction temperatures were 150–170 °C, the alkaline hydrolysis reaction, caused by the high temperature and strongly alkaline conditions, started at around 120 °C [23]. These authors also argued that hydrolysis started at just above 100 °C, and it produced not only vanillin but also vanillic acid, traces of acetovanillone, and other compounds. Finally, the lignin itself is a variable with a huge influence on the final yields of oxidation products. Considering lignin content in the reaction medium, Fargues et al. found that the vanillin yield only increased for lignin concentration of up to 60 g/L, decreasing for higher values [8]. Moreover, lignin with a low molecular weight and a less condensed structure tends to give better oxidation results, the presence of residual sugars is highly unfavorable, and the fewer structural transformations or chemical treatments lignin suffers, the better the reactivity toward oxidation and consequently the better the yields of phenolic compounds obtained [5][24][18].
The effect of one or several process parameters on lignin oxidative depolymerization performed through batch experiments has been intensively investigated with the primary objective of achieving the ideal conditions for obtaining the maximum conversion of lignin while avoiding the oxidation of the phenolic monomers produced [8,41,43,47,48,54,55,59]. The origin, composition, and processing of lignin (pulping process, isolation method, pretreatment, etc.), the oxygen partial pressure, the initial temperature, and the lignin and sodium hydroxide concentration in the reaction solution were studied, and its effect on the selectivity and efficiency of alkaline oxidative depolymerization process was evaluated [8,48,51,56,59,60]. It was found that a high partial pressure of oxygen reduces reaction time but leads to an increased rate of vanillin oxidation. Increasing the oxygen pressure accelerated both product formation and degradation, and therefore shortened the time needed to reach the maximum product yields [43,61]. In similar works, Schutyser and coworkers found that lignin oxidation under an inert atmosphere produced mainly oligomeric products, while the same reaction under oxygen primarily generated monomeric products [46]. Concerning the effect of pH of the mixture, it was concluded that during the lignin oxidation process, the yield of vanillin decreased when the pH value began to decrease. Moreover, there was a smaller vanillin degradation for strong alkaline conditions that increased significantly when pH was smaller than 11.5 [41,47]. Consequently, high alkali concentrations (pH > 12) are needed to reduce vanillin degradation. For temperature, it was found that an increase in this reaction condition can shorten the reaction time but, on the other hand, results in faster degradation of the aldehydes produced. However, Pacek and coworkers verified that even if usual reaction temperatures were 150–170 °C, the alkaline hydrolysis reaction, caused by the high temperature and strongly alkaline conditions, started at around 120 °C [62]. These authors also argued that hydrolysis started at just above 100 °C, and it produced not only vanillin but also vanillic acid, traces of acetovanillone, and other compounds. Finally, the lignin itself is a variable with a huge influence on the final yields of oxidation products. Considering lignin content in the reaction medium, Fargues et al. found that the vanillin yield only increased for lignin concentration of up to 60 g/L, decreasing for higher values [48]. Moreover, lignin with a low molecular weight and a less condensed structure tends to give better oxidation results, the presence of residual sugars is highly unfavorable, and the fewer structural transformations or chemical treatments lignin suffers, the better the reactivity toward oxidation and consequently the better the yields of phenolic compounds obtained [39,45,47].
Using the experimental results from evaluating the main reaction conditions’ effect in the alkaline oxidation with oxygen in a batch reactor, the authors developed a kinetic study of vanillin production [8][14][17][21]. The objective was to measure the reaction orders regarding lignin, oxygen, and vanillin species, as well as the influence of temperature on the kinetic rate constants to discuss the overall process of lignin oxidation [8]. The mathematical model proposed by the authors showed to be able to predict the behavior of vanillin oxidation for the different operating conditions tested. Since the vanillin produced by lignin oxidation is also oxidized and depends on the pH and the temperature of the solution, the influence of these two parameters on the kinetics of vanillin degradation had been studied on vanillin alone. The validation of a kinetic model for vanillin degradation separately confirmed that its degradation can be well predicted in the lignin oxidation experiments [14]. More recently, the kinetic model developed for vanillin degradation was improved by evaluating the degradation of all the main phenolic monomers produced from lignin oxidation: vanillin, vanillic acid, acetovanillone, syringaldehyde, syringic acid, and acetosyringone [17]. The kinetic study considering these individual products is of great interest since; during oxidation, their formation can be simultaneously accompanied by their degradation process that is dependent on the applied oxidation conditions. In
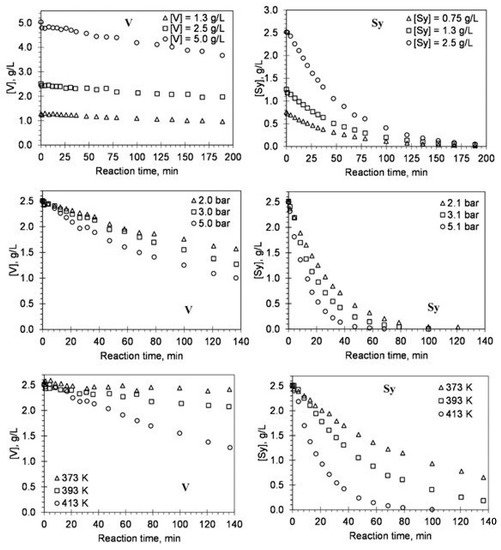
Using the experimental results from evaluating the main reaction conditions’ effect in the alkaline oxidation with oxygen in a batch reactor, the authors developed a kinetic study of vanillin production [48,56,59,61]. The objective was to measure the reaction orders regarding lignin, oxygen, and vanillin species, as well as the influence of temperature on the kinetic rate constants to discuss the overall process of lignin oxidation [48]. The mathematical model proposed by the authors showed to be able to predict the behavior of vanillin oxidation for the different operating conditions tested. Since the vanillin produced by lignin oxidation is also oxidized and depends on the pH and the temperature of the solution, the influence of these two parameters on the kinetics of vanillin degradation had been studied on vanillin alone. The validation of a kinetic model for vanillin degradation separately confirmed that its degradation can be well predicted in the lignin oxidation experiments [56]. More recently, the kinetic model developed for vanillin degradation was improved by evaluating the degradation of all the main phenolic monomers produced from lignin oxidation: vanillin, vanillic acid, acetovanillone, syringaldehyde, syringic acid, and acetosyringone [59]. The kinetic study considering these individual products is of great interest since; during oxidation, their formation can be simultaneously accompanied by their degradation process that is dependent on the applied oxidation conditions. In Figure 3, the effect of initial concentration, oxygen partial pressure, and temperature on the degradation as a function of reaction time for vanillin (V) and syringaldehyde (Sy) is shown [17].
, the effect of initial concentration, oxygen partial pressure, and temperature on the degradation as a function of reaction time for vanillin (V) and syringaldehyde (Sy) is shown [59].
Figure 3.
Effect of initial concentration, oxygen partial pressure, and temperature on the degradation as a function of reaction time for V and Sy. The lines represent the fitted kinetic model (experimental conditions: [NaOH] = 80 g/L; p
t = 9.8 bar) (reprinted with permission from [17]. Copyright 2019 American Chemical Society).
= 9.8 bar) (reprinted with permission from [59]. Copyright 2019 American Chemical Society).
All the performed studies allowed us to confirm that a trade-off between enhancing the phenolics conversion and minimizing oxidation is achieved for a temperature of around 120 °C, oxygen partial pressure around 3 bar, and lignin concentration of 60 g/L, prepared in a solution of 2 N NaOH [25][3][6][8]. Having these conditions as a starting point for the oxidative depolymerization of lignin, the LSRE team studied the potential of a wide variety of lignins from different origins and delignification processes. The yields of vanillin and syringaldehyde achieved for each lignin by nitrobenzene oxidation and alkaline oxidation with oxygen in the batch reactor are summarized in
All the performed studies allowed us to confirm that a trade-off between enhancing the phenolics conversion and minimizing oxidation is achieved for a temperature of around 120 °C, oxygen partial pressure around 3 bar, and lignin concentration of 60 g/L, prepared in a solution of 2 N NaOH [2,8,43,48]. Having these conditions as a starting point for the oxidative depolymerization of lignin, the LSRE team studied the potential of a wide variety of lignins from different origins and delignification processes. The yields of vanillin and syringaldehyde achieved for each lignin by nitrobenzene oxidation and alkaline oxidation with oxygen in the batch reactor are summarized in Table 1
. In addition to vanillin and syringaldehyde, other phenolics such as vanillic acid, acetovanillone, syringic acid, and acetosyringone were also identified in minor quantities; their occurrence also has a significant role in the study of reaction efficiency. Moreover, the presented data allow the evaluation of the benefit of lignin source and isolation on product yields.
Table 1.
Yields of vanillin and syringaldehyde obtained by nitrobenzene oxidation (NO) and alkaline oxidation using O
2
of lignins studied at LSRE (experimental conditions of alkaline oxidation using O
2
and performed in reactor batch: T
i
= 120 °C; [lignin] = 60 g/L; [NaOH] = 80 g/L; pO
2
= 3 bar; p
t
= 9.8 bar).
| Vanillin, % w/w lignin * |
Syringaldehyde, % w/w lignin * |
| |
NO ** |
Alkaline Oxid. |
NO ** |
Alkaline Oxid. |
| LInAT 1,2 |
9.3 |
3.4 |
- |
- |
| LWest 1 |
12.1 |
4.4 |
- |
- |
| LBoostS 1 |
11.1 |
3.1 |
- |
- |
| LOrgs 1 |
4.8 |
1.2 |
13.2 |
2.5 |
| KL 3 |
2.9 |
0.73 |
12.5 |
1.9 |
| KLlig 3 |
3.4 |
1.2 |
13.6 |
2.8 |
| EKL 3 |
2.2 |
0.71 |
9.2 |
1.4 |
| EKLlig 3 |
2.5 |
0.82 |
9.5 |
2.0 |
| HTEKL 3 |
3.4 |
0.54 |
9.8 |
1.5 |
| HTEKLlig 3 |
2.6 |
0.94 |
9.8 |
2.0 |
| SL 3 |
2.5 |
1.5 |
11.3 |
3.3 |
| LTobObut 4 |
2.8 |
0.74 |
2.5 |
0.34 |
| LTobOethan 4 |
7.2 |
1.2 |
4.8 |
0.94 |
| LCelbi |
1.7 |
0.81 |
9.5 |
2.1 |
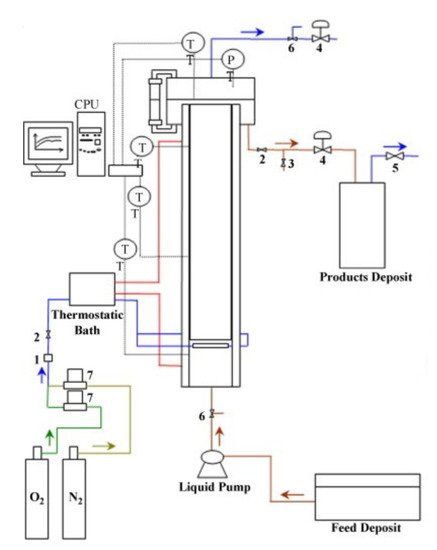
Most of the works focused on lignin oxidation have been performed in batch mode. However, from an industrial point of view, the continuous process of lignin oxidation presents more advantages due to the large volumes of liquor generated, the easier control of the process, and the lower overall investments and operating costs [13]. Araújo [14] built an experimental pilot setup to promote lignin oxidation in a continuous operating mode. The schematic diagram of the pilot installation is shown in
Most of the works focused on lignin oxidation have been performed in batch mode. However, from an industrial point of view, the continuous process of lignin oxidation presents more advantages due to the large volumes of liquor generated, the easier control of the process, and the lower overall investments and operating costs [55]. Araújo [56] built an experimental pilot setup to promote lignin oxidation in a continuous operating mode. The schematic diagram of the pilot installation is shown in Figure 4
. The bubble column reactor was made in 316L stainless steel with 8 L capacity, and the gas–liquid reaction takes place in the cylindrical body of the reactor. It has a 10 cm internal diameter and 70 cm height and is filled with three modules of Mellapak 750.Y structured packing (Sulzer Chemtech, Switzerland) that enhance the system’s overall mass transfer performance.
Figure 4. Schematic diagram of the pilot setup for the continuous production of phenolic monomers from lignin: (1) safety valve; (2) on–off valve; (3) electromagnetic valve; (4) needle valve; (5) safety valve; (6) three-way valve; (7) mass flow controller; PT—pressure transducer; TT—thermocouple. (Reprinted from [13] Copyright 2009, with permission from Elsevier.)
Schematic diagram of the pilot setup for the continuous production of phenolic monomers from lignin: (1) safety valve; (2) on–off valve; (3) electromagnetic valve; (4) needle valve; (5) safety valve; (6) three-way valve; (7) mass flow controller; PT—pressure transducer; TT—thermocouple. (Reprinted from [55] Copyright 2009, with permission from Elsevier.)
However, oxidation in the continuous reactor showed that the lignin conversion was substantially lower than that obtained for the batch process [14][15][16]. To improve the performance of the continuous reactor and reach the production yields obtained in bath mode, some studies focused on the influence of the main reaction were performed [13][14][20]. The results showed that the oxygen mass transfer from the gas phase to the reaction medium of sodium hydroxide and lignin was the limiting step to vanillin formation, and the use of pure oxygen in the gas feed was considered. In this case, the liquid residence time was decreased as the oxygen mass transfer rate increased to avoid excessive vanillin oxidation. A value of vanillin yield, in the exit stream, of approximately 85% of the maximum value obtained in the batch reactor was achieved considering the improvements in the continuous reaction [14][20].
However, oxidation in the continuous reactor showed that the lignin conversion was substantially lower than that obtained for the batch process [56,57,58]. To improve the performance of the continuous reactor and reach the production yields obtained in bath mode, some studies focused on the influence of the main reaction were performed [55,56,60]. The results showed that the oxygen mass transfer from the gas phase to the reaction medium of sodium hydroxide and lignin was the limiting step to vanillin formation, and the use of pure oxygen in the gas feed was considered. In this case, the liquid residence time was decreased as the oxygen mass transfer rate increased to avoid excessive vanillin oxidation. A value of vanillin yield, in the exit stream, of approximately 85% of the maximum value obtained in the batch reactor was achieved considering the improvements in the continuous reaction [56,60].
2. Dicarboxylic Acids from Lignin Oxidation
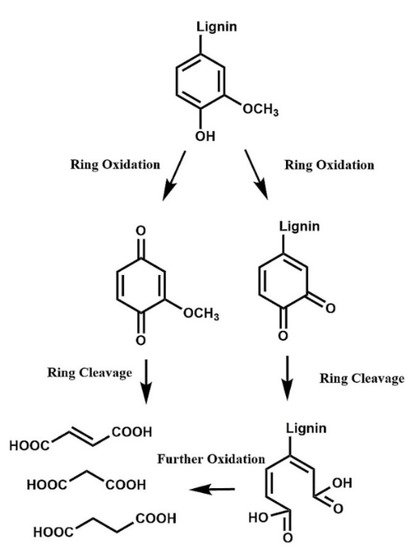
A harsh depolymerization causes cleavage of the remaining bonds that were not broken in mild depolymerization [27]. When the aromatic ring is cleaved (
A harsh depolymerization causes cleavage of the remaining bonds that were not broken in mild depolymerization [11]. When the aromatic ring is cleaved ( Figure 5
), C
6
acids are obtained (mainly muconic acid), which are quickly degraded to lower carbon-content acids (C
2
-C
4 acids) [28]. The products can be completely mineralized to CO
acids) [12]. The products can be completely mineralized to CO 2
and H
2
O under very harsh conditions. Even though C
6
acids have important industrial uses, they are very unstable and are swiftly converted to C
4
dicarboxylic acids (C
4
-DCA), which are relatively stable and can be easily separated at the end of the reaction.
Figure 5.
Oxidation of lignin through ring-opening reactions to yield C
4 dicarboxylic acids. (Reprinted from [29] Copyright 2018, with permission from Elsevier.)
dicarboxylic acids. (Reprinted from [64] Copyright 2018, with permission from Elsevier.)
The acids with a higher prevalence in lignin oxidation are succinic (SA), malic (MAL), and maleic acids (MA), with small amounts of fumaric (FA) and tartaric (TA) acids. Most of these acids are currently used in food, pharmaceutical, and polymer industries, as well as chemical precursors for 1,4-butanediol, tetrahydrofuran, and γ-butyrolactone [30][31][32][33][34][35][36][37][38]. In the last 15 years, several authors have studied C
The acids with a higher prevalence in lignin oxidation are succinic (SA), malic (MAL), and maleic acids (MA), with small amounts of fumaric (FA) and tartaric (TA) acids. Most of these acids are currently used in food, pharmaceutical, and polymer industries, as well as chemical precursors for 1,4-butanediol, tetrahydrofuran, and γ-butyrolactone [13,14,15,65,66,67,68,69,70]. In the last 15 years, several authors have studied C 4
-DCA from lignin and lignin model compounds under catalytic and non-catalytic conditions, using different strong oxidants, i.e., O
2
, O
3
, H
2
O
2,
and peracetic acid. Some of these works were focused on lignin depolymerization towards aromatic monomers and reported the C
4
-DCA as a degradation product. Yet, this information is valuable to identify possible research lines and optimal conditions for lignin depolymerization.
2.1. Non-Catalytic Harsh Oxidation
Even though O
2
is widely used for lignin depolymerization to phenolics, it can cause ring-opening reactions. However, its oxidant power is lower than other oxidants, being less effective towards C
4-DCA [39][40][41][42]. Demesa et al. [41] performed alkali lignin oxidation using O
-DCA [16,36,71,72]. Demesa et al. [71] performed alkali lignin oxidation using O 2, achieving up to 3 wt% of succinic acid (SA). Ozone, a stronger oxidant, was used on pyrolytic lignin, obtaining a small amount of SA (2.0 wt%) and maleic acid (MA) (2.3 wt%) [43]. Comparatively, previous works from other research groups using O
, achieving up to 3 wt% of succinic acid (SA). Ozone, a stronger oxidant, was used on pyrolytic lignin, obtaining a small amount of SA (2.0 wt%) and maleic acid (MA) (2.3 wt%) [73]. Comparatively, previous works from other research groups using O 3
on technical lignins showed low yields of C
4-DCA [44][45].
Hydrogen peroxide has received a strong focus when ring-opening reactions are the objective because it is more reactive than O
2, with the benefit of being environmentally benign, allowing milder conditions, and avoiding mass transfer barriers that appear between liquid and gas phases [4][46][47][48]. However, given that H
, with the benefit of being environmentally benign, allowing milder conditions, and avoiding mass transfer barriers that appear between liquid and gas phases [22,76,77,78]. However, given that H 2
O
2 is a weak acid, its reactivity is strongly associated with the pH, being stable at acidic conditions but decomposing in alkaline conditions [49][50]. Lignin model compounds (guaiacol, syringol, and phenol) were oxidized using H
is a weak acid, its reactivity is strongly associated with the pH, being stable at acidic conditions but decomposing in alkaline conditions [79,80]. Lignin model compounds (guaiacol, syringol, and phenol) were oxidized using H 2
O
2
at 300 °C and short times, obtaining different C
1
-C
6 dicarboxylic acids [51]. Catechol oxidation reached very high yields of TA, FA, and MAL, with up to 41% C
dicarboxylic acids [81]. Catechol oxidation reached very high yields of TA, FA, and MAL, with up to 41% C 4-DCA [49]. Both studies concluded that the oxidation of the phenolic model compounds goes through
-DCA [79]. Both studies concluded that the oxidation of the phenolic model compounds goes through o
-benzoquinones and
p
-benzoquinones, yielding muconic and 2,5-dioxo-3-hexenoic acids, which are highly unstable and are degraded to C
4
-DCA. Some C
4-DCA were identified in hardwood kraft lignin oxidation using peracetic acid [52]. Flow reactor oxidation of alkali lignin using H
-DCA were identified in hardwood kraft lignin oxidation using peracetic acid [82]. Flow reactor oxidation of alkali lignin using H 2
O
2 showed up to 13 wt% SA [53] when using very high temperatures and short times. SA was formed above 150 °C, confirming that harsh conditions are required to achieve valuable yields, at least in a non-catalyzed reaction.
showed up to 13 wt% SA [83] when using very high temperatures and short times. SA was formed above 150 °C, confirming that harsh conditions are required to achieve valuable yields, at least in a non-catalyzed reaction.
Following the interest in the C
4-DCA obtained from lignin, the LSRE group analyzed the peroxide oxidation of lignin and lignin model compounds [54], studying the effect of the methoxy substituents in the lignin aromatic ring on the production of C
-DCA obtained from lignin, the LSRE group analyzed the peroxide oxidation of lignin and lignin model compounds [84], studying the effect of the methoxy substituents in the lignin aromatic ring on the production of C 4
-DCA. It was found that methoxy substituents increased reactivity toward peroxide oxidation, causing lignin model compounds with more methoxy substituents (syringic acid) to be degraded easily, while
p
-hydroxybenzoic acid (without methoxy substituents) was more resistant to oxidation. Compounds with lower methoxy substituents (
p
-hydroxybenzoic and vanillic acid) showed higher overall C
4
-DCA yield and higher succinic acid (SA) yield than syringic acid. Interestingly, when two lignins with different S:G ratios were compared, the hardwood lignin (higher S:G ratio) not only showed a better lignin conversion but also achieved a higher SA yield (3.2 wt%). The softwood lignin achieved a lower SA yield (2.5 wt%) but a higher MAL yield in the first minutes of the reaction. This study demonstrated that even though the methoxy substituent can reduce C
4
-DCA production from model compounds, they boost lignin reactivity towards peroxide oxidation, making it easier to depolymerize lignin into smaller fragments that will be converted to C
4
-DCA, increasing their final yield.
2.2. Catalytic Lignin Harsh Oxidation
Peroxide oxidation of lignin towards C
4-DCA can be performed using different types of catalyst, which can vary from expensive noble metals, cheaper zeolites, or even homogeneous metal ions, such as Fenton’s reagent [41]. More than 65% of the latest publications on lignin conversion to C
-DCA can be performed using different types of catalyst, which can vary from expensive noble metals, cheaper zeolites, or even homogeneous metal ions, such as Fenton’s reagent [71]. More than 65% of the latest publications on lignin conversion to C 4
-DCA use catalytic conversion, and those works were focused on two oxidants: O
2
and H
2
O
2
, with the latter having a higher amount of research.
Homogeneous catalysts are mainly transition metal ions with at least two oxidation states, e.g., Cu
+/2+
and Fe
2+/3+
. Oxygen oxidation with these catalysts produced very low C
4-DCA yields [55][56]. With H
-DCA yields [85,86]. With H 2
O
2 in Fenton’s conditions, phenol oxidation yielded 8% of MA [57], and other model compounds produced small amounts of MA and FA (<2%) [58]. However, no C
in Fenton’s conditions, phenol oxidation yielded 8% of MA [87], and other model compounds produced small amounts of MA and FA (<2%) [88]. However, no C 4-DCA was obtained when lignin was oxidized [59], concluding that Fenton’s catalyst approach is not efficient for depolymerization towards C
-DCA was obtained when lignin was oxidized [89], concluding that Fenton’s catalyst approach is not efficient for depolymerization towards C 4
-DCA.
Different heterogeneous catalysts have been used with distinct outcomes. Perovskite-type oxides (such as chalcopyrite) have in their structures transition metals with at least two different oxidation states, which catalyzes H
2
O
2 oxidation [60][61]. Chalcopyrite (CuFeS
oxidation [42,90]. Chalcopyrite (CuFeS 2) presented promising results in model compounds [28] and biorefinery lignins (diluted-acid corn stover lignin: 7% SA, 1% MAL), but with low SA yields for bagasse lignin [62]. Chalcopyrite nanoparticles used on lignin at acidic pH produced high yields of SA (12%) with low yields of FA and MA (1%, each) [63]. Other catalysts, such as sodium percarbonate in alkaline conditions, yielded ~1% SA and MA/FA traces for bagasse oxidation [62]. Gas-phase O
) presented promising results in model compounds [12] and biorefinery lignins (diluted-acid corn stover lignin: 7% SA, 1% MAL), but with low SA yields for bagasse lignin [91]. Chalcopyrite nanoparticles used on lignin at acidic pH produced high yields of SA (12%) with low yields of FA and MA (1%, each) [92]. Other catalysts, such as sodium percarbonate in alkaline conditions, yielded ~1% SA and MA/FA traces for bagasse oxidation [91]. Gas-phase O 2 oxidation using aluminium-vanadium-molybdenum oxide and vanadium pyrophosphate in a fluidized bed only produced small amounts of MA (1.5 wt%) [64], while eight supported metal catalysts (involving V, Mo, Mg, and W) produced a small amount of C
oxidation using aluminium-vanadium-molybdenum oxide and vanadium pyrophosphate in a fluidized bed only produced small amounts of MA (1.5 wt%) [93], while eight supported metal catalysts (involving V, Mo, Mg, and W) produced a small amount of C 4-DCA (SA and/or MA/FA) [65]. It was V-W/HZSM-5 that produced the highest yields (nearly 2% SA and 12% MA/FA), confirming that V
-DCA (SA and/or MA/FA) [94]. It was V-W/HZSM-5 that produced the highest yields (nearly 2% SA and 12% MA/FA), confirming that V 5+
activates the aromatic rings in monomeric units.