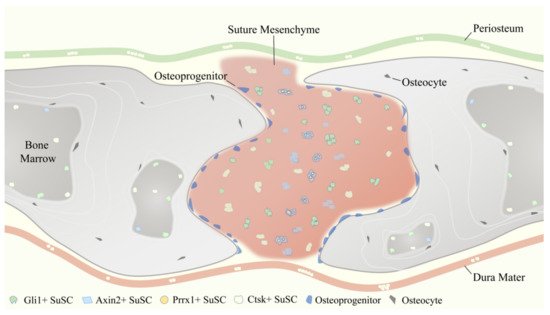
SuSCs play an indispensable role in the injury repair and tissue regeneration of calvarial bone defects after birth
[30][58]. Studies have shown that Gli1+ SuSCs were rapidly activated into proliferation within 24 h after an injury occurs. Two weeks after experimental injury, most of the infiltrated cells within the injury site were labeled, indicating their derivation from Gli1+ SuSCs. One month after experimental injury, the periosteum, dura mater, and osteocytes in the re-ossified region were labeled, suggesting that Gli1+ SuSCs contribute to calvarial bone defect repair
[6][1]. Additionally,
Gli1-CreERT2;R26-ZsGreenflox mice were induced and their calvaria (skull bone flaps containing the sagittal suture) were dissected under sterile conditions and transplanted into nude mice, which were used as the recipient mice with a calvarial window for placing transplants. It was found that the suture transplants integrated into the host bone and healing were achieved one-month post-surgery, with a significant number of cells within the periosteum, dura mater, and bone of the transplant strongly labeled. On the contrary, transplants not containing any suture tissue (with periosteum and dura mater preserved) from the same donor mice were transplanted and served as controls and ended up with poor healing and failure in generating new periosteum, dura mater, or osteocytes
[6][1]. Thus, the cranial sutures and the resident Gli1+ SuSCs are the main sources of reparative cells functioning in calvarial bone injury repair; the periosteum and dura mater are either unable or insufficient to accomplish efficient calvarial bone regeneration. Similarly, Axin2+ SuSCs also respond to calvarial bone injury and promptly expand within the suture mesenchyme. Four weeks after experimental injury, a drastic expansion of Axin2+ SuSCs has been observed surrounding the skeletogenic suture mesenchyme. Further, Axin2+ SuSCs moved into the injury site and co-localized with Osx+ osteoprogenitors and Sost+ osteocyte, indicating their direct contribution to the injury repair of the skull. When Axin2+ SuSCs were isolated from
Axin2Cre-Dox;R26RlacZ mice and directly implanted into the injury site, enhancements of the healing process were detected at two and four weeks after the operation. In comparison, neither transferring Axin2
− cells nor implanting without any cells serving as control did not show significant improvement
[7][2]. As expected, 5 days, 10 days, and 30 days after experimental injury, Prrx1+ SuSCs and their progenies were found to contribute to the repair and regeneration of neural crest-derived (frontal) and mesoderm-derived (parietal) calvarial bones
[8][3]. Plus, the parietal bone defects were unable to heal if the surrounding coronal and sagittal sutures were surgically removed concomitantly to the creation of the defect, while removal of the sutures away from the parietal bone defects did not affect the healing process
[8][3]. Regarding Ctsk+ SuSCs, it has not been tested through the calvarial bone defect model to evaluate their performance in injury repair. However, based on the pivotal role of Ctsk+ PSCs in the process of long bone fracture healing
[9][4], we speculate that Ctsk+ SuSCs should facilitate calvarial bone healing as well.