Inflammation ihas been reported to be intimately linked to the development or worsening of several non-infectious diseases. CA number of chronic conditions such as cancer and, diabetes, cardiovascular disorders often, autoimmune diseases, and neurodegenerative disorders emerge as a result fromof tissue injury and genomic changes induced by persisteconstant low-grade inflammation. Current treatments for these diseases are often not curative and come with significant side effects. Apigenin, a flavonoid found in common fruits and vegetables, has garnered attention for its broad biological activities, including anti-inflammatory, antioxidant, and anti-cancer properties. Despite its potential, achiev in and around the affected tissue or organ. The existing therapies for most of these chronic conditions sometimes leave more debilitating effects than the disease itself, warranting therapeutic levels of apigenin, especially in the CNS, remains challenging due to its limited bioavailability and rapid metabolism. Recent research has focused on developing advanced delivery systems, such as nanosized drug delivery systems, enteric polymer-coated spheres, and intranasal formulations, to enhance its bioavailability a advent of safer, less toxic, and more cost-effective therapeutic alternatives for the patients. For centuries, flavonoids and therapeutic efficacy. These innovative delivery methods show promise in maximizing apigenin's potential as a therapeutic agent for chronic inflammatory diseases, metabolic syndrome, cardiovascular diseases, cancer, and neuroinflammatory disorderir preparations have been used to treat various human illnesses, and their continual use has persevered throughout the ages.
1. Role Introductioof Apigenin as an
As predicted by the World Economic Forum, within the next 16 years, management of chronic disease including neuroinflammation is predicted to cost the world a staggering $47 trillion in treatment and lost wages. The treatments currently available are rarely curative and have serious side effects. The use of plant-based substances for the treatment of various mental ailments has been prevalent for centuries [1]. Of these, flavonoids are an important group of more than 4000 polyphenolic compounds possessing a common phenylbenzopyrone structure (C6-C3-C6), which allows a wide range of biological activities [2][3]. Among other related flavonoids, apigenin, a naturally occurring plant flavone, is found in abundance in common fruits and vegetables such as parsley, tea, chamomile, wheat sprouts, and some seasonings. It represents about 0.8% of the total flavonoids consumed on a daily basis by the U.S. population, estimated by the department of food science and human nutrition [4].
2. Chemical Properties and Bioavailability
Apigenin is a low molecular weight flavone (molecular weight = 270.24 kDa), practically insoluble in water, partially soluble in hot alcohols, and completely soluble in potassium hydroxide (KOH) and dimethylsulfoxide (DMSO). Apigenin is a major constituent of chamomile and when prepared as chamomile tea, it has been used for centuries as a natural remedy for relieving indigestion and gastritis. Chamomile preparations have also been traditionally used in skin care products for the treatment of cutaneous inflammation and other skin disorders [5].
For the successful development of any natural product lead compound as a therapeutic entity, several important characteristics such as increased bioavailability, long half-life, and slow absorption and excretion need to be fulfilled. Additionally, the bioactive molecule should be able to convene at the target organ at an effective concentration unaltered, for it to exert a suitable biological effect. The bioavailability of a number of flavonoids has been extensively studied and the results have shown rapid excretion and extensive metabolism following ingestion.
Most of the flavonoids are detected in the blood stream within a few minutes to a few hours, with half-lives not exceeding 48 h, after which they are excreted from the body. However, apigenin shows comparatively slower absorption kinetics and delayed plasma clearance in pathogen-free Wistar rats after single oral administration [6]. Apigenin is seen in the blood stream only 24 h after ingestion, with a relatively longer half-life of 91.8 h. Apigenin is also recovered at basal levels up to 10 days post ingestion, indicating slow excretion kinetics due to slow decomposition in the liver. These remarkable pharmacokinetics warrant further studies in human subjects to ascertain bioactive concentrations of apigenin required to obtain the anti-inflammatory pharmacological effects that have been reported in vitro.
3. ProAntective Effects of Apigenin
3.1 Anti-Inflammatory Agent
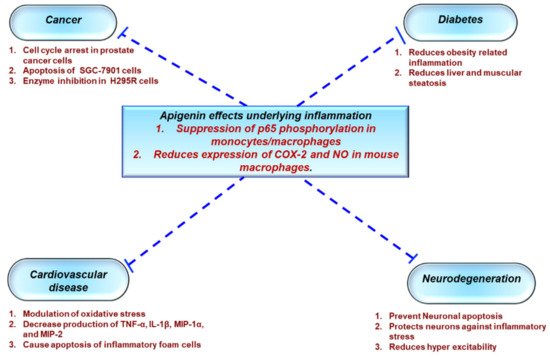
As the health effects of polyphenols depend on intake and bioavailability, the biological activities of apigenin (4’,5,7-trihydroxyflavone), an abundantly occurring flavone have, been extensively studied
[7][8][134,135]. Much like the family to which it belongs, apigenin possesses a wide array of biological properties including anti-oxidant, anti-cancer, and anti-inflammatory actions
[9][10][5][136,137,138]. As a result, apigenin has gained a lot of interest in the past few years as a potential therapeutic agent to treat various diseases such as cancer, diabetes, cardiovascular, and neurological disorders
[11][139] (
Figure 12).
Figure 12. Role of apigenin in chronic inflammatory diseases. Apigenin as an anti-inflammatory compound acts as a protective agent in several disorders via inhibition of key inflammatory mediators, signaling pathways, and molecules. COX-2—cyclooxygenase-2; IL—interleukin; TNF—tumor necrosis factor; NO—nitric oxide.
3.2 Metabolic Syndrome
Metab
1.1. Protective Effects of Apigenin Across a Spectrum Of Chronic Diseases
Acco
lic syndr
ome, as defined byding to the National Cholesterol Education Program’s Adult Treatment Panel III (NCEP: ATP III),
is metabolic syndrome is associated with abdominal obesity, dyslipidemia, hyperglycemia, inflammation, insulin resistance, or diabetes mellitus, as well as an increased risk of developing cardiovascular disease. Obesity is attributed to adipose tissue dysfunction and expanded adipose tissue mass, which can lead to the upregulation of proinflammatory cytokines such as TNF-α and IL-6, resulting in a state of chronic low-grade inflammation
, as previously discussed. Apigenin has been shown to inhibit an important inflammatory biomarker, CD38, in a metabolic syndrome model, as well as decrease adipose tissue mass and the levels of proinflammatory cytokines
[12][140]. Additionally,
Feng et a
pl. have reportedly shown that apigenin improves obesity and obesity induced inflammation
[13][141]. Apigenin has also been reported to attenuate inflammation and the resultant pathological alterations in rats fed with a high fat, fructose diet
[14][142]. In diabetic rats, apigenin reduces metabolic inflammation by successfully polarizing infiltrating macrophages to an anti-inflammatory M2 phenotype by binding and activating PPAR-γ and the subsequent suppression of the NF-κB pathway
[13][141]. Apigenin also ameliorated renal dysfunction in diabetic rats by suppressing inflammation through reduced secretion of TNF-α and IL-6 via MAPK inhibition. Histopathology confirmed reduced inflammation in the renal tissue along with reduction in collagen deposition and glomerulosclerosis
[15][143].
3.3 Ulcerative Colitis
Apigenin served as a potent therapy against
ulcerativeUC colitis iin C57BL/6 mice through the inhibition of inflammatory cytokines,
and COX-2, and through the reduction in immune cell infiltration in colon tissues
[16][144]. Because NF-κB activation upregulates epithelial cell permeability, promoting colonic inflammation, testing apigenin effect in vitro on colon carcinoma cells HCT-116 demonstrated NF-κB downregulation in a dose-dependent manner. Apigenin also reduced levels of matrix metalloproteinase (MMP-3), which aids in extracellular remodeling, contributing to colonic inflammation, thereby showing protective effects in a murine DSS (dextran sulphate sodium) colitis model
[17][145]. The use of a soluble form of apigenin showed amelioration of in colitis models in rats through the inhibition of various inflammatory markers such as TNF-α, transforming growth factor-b, IL-6, intercellular adhesion molecule 1, or chemokine (C–C motif) ligand 2
[18][146].
3.4 Non-Alcoholic Fatty Liver Disease (NAFLD)
Inflammation in NAFLD is one of the main causes of insulin resistance with inflammatory markers such as TNF-α and IL-6 suppressing insulin receptor signaling, thus blocking the action of insulin in hepatocytes. In NASH mice fed with a high fat diet, apigenin ameliorated inflammation through reduction of plasma levels of MCP-1, IFN-γ, TNF-α, and IL-6
[19][147].
3.5 Cardiovascular Diseases
Beneficial aspects of apigenin activity help to ameliorate inflammation-mediated cardiac injury, indicating a role for apigenin as a therapeutic agent against cardiovascular diseases. In an LPS-induced model of myocardial injury, apigenin relieved injury by modulating both oxidative stress and inflammatory cytokines such as TNF-α, IL-1β, MIP-1α, and MIP-2 through NF-κB regulation
[20][148]. Macrophages loaded with oxidized LDLs contribute significantly towards the progression of atherosclerotic plaques. Apigenin was shown to induce apoptosis of murine peritoneal macrophages through reduction in expression of anti-apoptotic plasminogen activator inhibitor 2
[21][149]. Apigenin can promote apoptosis in foam cells through inhibition of autophagy and subsequently reduce the foam-cell mediated secretion of proinflammatory cytokines during atherogenesis
[22][150]. Additionally, apigenin helped in cholesterol efflux from macrophages in atherosclerotic lesions in apoE
−/− mice challenged with LPS through the increased expression of ATP binding cassette A1 (ABCA1) and reduced expression of proinflammatory cytokines, and reduced levels of NF-κB and TLR-4
[23][151].
3.6 Anticancer
Several studies have investigated the anti-cancer effects of apigenin and shown its ability to suppress cancer cell proliferation in various types of tumors, including pancreatic, colorectal, liver, blood, lung, cervical, prostate, breast, thyroid, skin, head, and neck
[24][25][26][152,153,154]. Because inflammatory molecules modulate the physiological and pathological states of cancer and its surrounding microenvironment, and tumor initiation is said to occur as a result of prolonged exposure to inflammatory conditions, inhibition of inflammatory molecules could be a promising approach to managing cancer
[27][155]. The NF-κB pathway and its associated molecules are key regulators of cancer cell survival and proliferation through increased expression of cell cycle related VEGF, inflammatory cytokines, and metastatic genes such as COX-2
[24][152]. Apigenin reduced prostate tumor volumes in mouse models through suppression of NF-κB activation
[28][156]. In non-small cell lung cancer cell line A549, apigenin blocks the nuclear translocation of NF-κB, thereby suppressing the expression of tumorigenic genes such as Bcl-2, Mcl-1, and Bcl-xL. Apigenin also inhibits several signaling pathways including NF-κB and MAPK, inducing anti-cancer effects in malignant mesothelioma
[29][157].
3.7 Neuroprotection
Apigenin, found abundantly in a variety of plants, herbs, and spices [9][30], has been utilized for centuries to treat diseases such as asthma, insomnia, Parkinson’s, neuralgia, and shingles [30][31], suggesting its potential use for both peripheral and CNS disorders. Apigenin has been shown to exert its neuroprotective effect via suppressing the expression of an inducible form of nitric oxide synthase (iNOS) and nitric oxide (NO) in microglial cells and macrophages [32]. Also, through regulation of adhesion molecules such as VCA M
-1, ICAM-1, a
nd E-selectin [33], whic
h play a cr
itical role in controlling leukocyte migration across the endothelial cells of BBB, apigenin might inhibit immune cells’ entry into the CNS and prevent neuroinflammation. However, it remains elusive whether apigenin or other flavones could serve as a potential treatment for neuroinflammatory disorders like multiple sclerosis, which affects approximately 400,000 people in the United States alone.4. Mechanism of Action
4.1 Anti-inflammatory Mechanisms
Macrophages are ophages are the most
he most abundant innate immune cells in the tumor microenvironment that contribute to chronic low-grade inflammation, leading to tumor growth and metastasis through tumor neovascularization and matrix remodeling
[34][158]. Apigenin induced apoptosis in mouse ANA-1 macrophage cell line through regulation of MAPK pathway and suppression of anti-apoptotic gene Bcl-2
[35][159]. Exposure to ultraviolet B (UVB) radiation results in acute inflammation due to production of various cytokines and chemokines via COX-2 expression and the resultant recruitment of neutrophils, monocytes, and macrophages, leading to acute responses such as skin edema or chronic inflammation, fibrosis, and cancer. Apigenin suppresses UVB-induced skin carcinogenesis through inhibition of inflammatory COX-2 and restoration of anti-angiogenic and anti-inflammatory thrombospondin-1
[36][160]. TNF-α contributes to breast cancer metastasis through the recruitment of tumor-infiltrating macrophages, neutrophils, and T cells, leading to immune evasion, tumor growth, and metastasis. Apigenin was shown to down-modulate TNF-α mediated upregulation of chemotactic protein, CCL2, granulocyte macrophage colony-stimulating factor (GM-CSF), IL-1α, and IL-6 in human triple-negative cells (MDA-MB-231 cells)
[37][161].
4.2 Neuroprotective Mechanism
T
Apigenin, found abundantly in a variety of plants, he
rbs, nand spices [136,162], has be
en u
roprotective efftilized for centuries to treat diseases such as asthma, insomnia, Parkinson’s, neuralgia, and shingles [162,163], sugge
csting its
of apotential use for both peripheral and CNS disorders. Apigenin
and its mechanism of action are not yet fully undehas been shown to exert its neuroprotective effect via suppressing the expression of an inducible form of nitric oxide synthase (iNOS) and nitric oxide (NO) in microglial cells and macrophages [131]. Also, thr
sough regulat
ood. To evion of adhesion molecules such as VCAM-1, ICAM-1, and E-selectin [164], which pla
luy a
te the therapeutic potential of critical role in controlling leukocyte migration across the endothelial cells of BBB, apigenin
in regulatingmight inhibit immune cells’ entry into the CNS and prevent neuroinflammation
, its efficacy was tested in experimental autoimmune encephalomyelitis (EAE). However, it remains elusive whether apigenin or other flavones could serve as a potential treatment for neuroinflammatory disorders like multiple sclerosis, which affects approximately 400,000 people in the United States alone. Very little is known about the neuroprotective effects of apigenin and its related mechanism of action. In order to assess the therapeutic potential of apigenin in regulating neuroinflammation, we tested its efficacy in EAE models of relapse-remitting
multiple sclerosis (MS). In these models, MS wherein apigenin reduced disease severity through inhibiting immune cell infiltration into the CNS and subsequent
ly reduc
ingtion in demyelination
[38][132].
MS is an autoimmune disease with an as yet unknown etiologic agent mediated by an immunogenic response of auto-inflammatory T cells against the myelin sheath protecting the neurons. Dysregulation of
dendritic cell (DC
) function in MS can result from several possible reasons, which include, but are not limited to T-cell anergy in response to persistent antigens displayed by long-lived lymphoid DCs and functional abnormality of DCs (
Figure 23). The infiltration of DCs from the periphery during neuroinflammatory autoimmunity has been studied extensively, particularly in EAE models of MS wherein DCs infiltrating from the blood increase with the increasing clinical severity of EAE. In fact, evidence shows that they interact with naive CD4
+ T cells, driving T
h17 differentiation, a T cell subset involved in chronic inflammatory disease
[39][40][165,166].
R Hence, the regulati
on
g of DC functions and
theirits transmigration into the CNS
is crucialholds the key to prevent the detrimental effects of immune infiltration in MS. Current MS therapies such as
nNatalizumab and dimethyl fumarate (DMF) that regulate leucocyte entry into the CNS have shown potential in controlling symptoms and relapse
[41][167]. However, most of these
therapies do not control the progressive form of the MS and are often associated with significant side effects, emphasizing the need for and value of identifying safer, alternate therapies that could provide clinical level benefits for the debilitating diseases of the CNS.
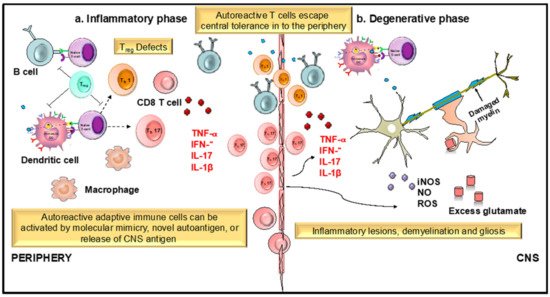
Figure 23. Role of dendritic cells (DCs) and T cells in the development and progression of multiple sclerosis (MS). MS is an immune mediated disease characterized by an initial inflammatory event consisting of presentation of as yet unknown antigens to CD8 T cells, their entry across the blood–brain barrier (BBB) into the central nervous system (CNS), and their subsequent reactivation by CNS resident DCs and microglial cells. This results in an inflammatory cascade involving secretion of several proinflammatory mediators such as cytokines IL-1β, IL-17, and TNF- α. The release of these cytokines initiates the degenerative phase that is characterized by increase in iNOS, NO, glutamate, and ROS, which brings about formation of inflammatory lesions, gliosis, and demyelination, which are the hallmarks of MS.
4.2. Apigenin Mediated Modulation in Dendritic Cell Phenotypical and Functional Maturation
1.2. Apigenin Mediated Modulation in Dendritic Cell Phenotypical and Functional Maturation
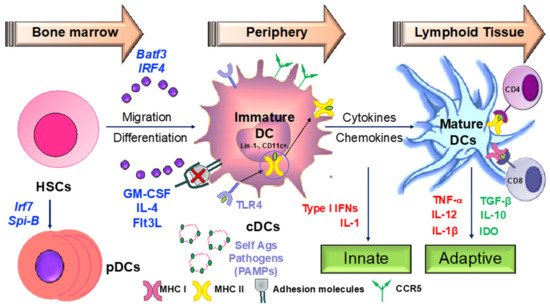
Hematopoeitic stem cells in the bone marrow differentiate into plasmacytoid
dendritic cells (pDCs
) from lymphoid progenitors in the presence of transcription factors such as like Irf7 and Spi-B
[42][168] (
Figure 34). DC progenitors
in the bone ma
rrow also give rise to circulating precursors in the presence of other factors like Batf3 and Irf4 that home to tissues, where they reside as immature cells with high phagocytic capacity
[43][169]. Following tissue damage, immature DCs capture antigens through
pattern recognition receptors (PRRs
) such as T
oll-like receptors (TLRs)LRs, and initiate the innate response through the secretion of IL-1 and type I interferons.
Following antigen capture, immature DCs also subsequently migrate to the lymphoid organs, where they select rare antigen-specific T cells, thereby initiating adaptive immune responses. T cells are also educated by DCs to recognize and tolerate self-antigens. Upon sSensing of microbial stimuli through PRRs, DCs undergo causes DCs to enter the process of maturation, which involves the upregulation of major histocompatibility complex (MHC) molecules and co-stimulatory molecules. Peptide-loaded MHC molecules are recognized by Ag-specific T cell actis via the T-cell receptor (TCR), constituting signal 1 of T cell activation involves:
- Signal 1: Peptide-loaded MHC molecules recognized by antigen-specific T cells via the T-cell receptor (TCR).
- Signal 2: Binding of costimulatory molecules on DCs to CD28/CD40L on T cells. Activated T cells in turn help DCs in terminal maturation through the ligation of CD40 and CD80/86.
- Signal 3: Release of inflammatory cytokines and chemokines promoting the differentiation of naïve antigen-specific T cells into effector cells and activation of various other immune cells by the dendritic cells.
Therapeutic agents targeting these steps involved in DC-mediated T cell activation may be critical in ameliorating various chronic inflammatory diseases.
. Signal 2 consists of binding of costimulatory molecules on DCs to CD28/CD40L on T cells. Activated T cells in turn help DCs in terminal maturation through the ligation of CD40 and CD80/86. The final step in T cell activation is signal 3; the release of inflammatory cytokines and chemokines promoting the differentiation of naïve antigen-specific T cells into effector cells, as well as the activation of various other types of immune cells by the dendritic cells. Therapeutic agents targeting the various steps involved in DC-mediated T cell activation may be critical in the amelioration of various chronic inflammatory diseases.
Figure 34. Dendritic cells as sentinels of the immune system. DCs orchestrate the immune response initiating both the innate and adaptive branches of the immune system. Any dysregulation in their activity is the key to development of chronic inflammatory and autoimmune conditions. IFN—interferon; GM-CSF—granulocyte macrophage colony-stimulating factor; HSC—hematopoietic stem cell(s); MHC—major histocompatibility complex; TLR—toll-like receptor.
Various flavonoids, described earlier in this review, inhibit the inflammatory functions of DCs. The role of apigenin on DC maturation and function has been investigated in murine bone marrow
- derived DCs
. Apigenin has linked to th, where inhibition of p65 translocatio
n, resulting in reducedn has been linked to downmodulation in cell surface expression of DC co-stimulatory molecules and antigen capture
[44][170].
In a collaApigen
-inin reduced
the severity of arthritis
mouse model, apigenin rein a collagen-induced
the severity of arthritis
mouse model by reducing proinflammatory cytokine secretion from serum and supernatants from lymph node DCs. DCs from the apigenin-treated mice also exhibited low expression of MHC and co-stimulatory molecules
[45][171].
More recently, apigenin was seen to reduce the expression of co-stimulatory CD80, CD86, and MHC II on murine splenic CD11c+ DCs. Additionally, LPS-matured splenic DCs pulsed with ovalbumin (OVA)323−339 and treated with apigenin impaired OVA-specific T cell proliferation
[46][172]. However, the molecular players involved in the apigenin mediated control of DC function are still mostly unknown.
It is also unclear whether apigenin is able to modulate DC phenotype and functional characteristics to regulate antigen-specific T cells in neuroinflammatory conditions. THence, we investigated the effects of apigenin was investigated in in EAE mice and reported disease attenuation and reduced demyelination. Amelioration in the disease phenotype was dictated by reduced CNS infiltration of myeloid immune cells. Functionality of both Th1 and Th17 cells was impaired and FoxP3+ Treg cell numbers were seen to be boosted in apigenin-treated EAE mice.
To evaluate whether these protective effects of apigenin are mediated by changes in DC phenotype and function, studies were conductedwe investigated the effects of apigenin on human peripheral blood DCs. Unpublished data sufrom these studies suggest that apigenin reduced cell surface expression of key antigen presentation and co-stimulatory markers and reduced the secretion of proinflammatory cytokines in LPS-matured DCs treated with apigenin in a RelB-dependent manner.
It is known that NF-κB activation is required for T-cell activation by DCs, primarily through the canonical NF-κB pathway
[47][173]. NF-κB consists of a family of five Rel proteins; namely, c-Rel, RelA/p65, RelB, NF-κB1 (p50 and its precursor, p105), and NF-κB2 (p52 and its precursor, p100), of which p65 and p50 predominantly compose the canonical pathway. Recent findings
have sincreasingly suggested a role
forof NF-κB protein RelB in DC maturation,
their antigen present
ationing functions, and DC-mediated immunity
[47][48][173,174]. In mature DCs, RelB is upregulated and translocated into the nuclei in response to various maturation signals
[49][175]. Additionally, the apigenin-induced changes in blood DCs lead to T-cell polarization away from T
h1 and T
h17 cells towards T
reg cells, as
observedwas seen in the
apigenin- EAE mice treated
EAE micewith apigenin. Thus, a DC-central anti-inflammatory agent
like apigenin could be key in resolving CNS inflammation and the resultant pathologies in various neurodegenerative diseases.
5
2. Development of Apigenin as a Viable Candidate for Anti-Neuroinflammatory Treatment
5.1 Synthesis and Derivatives
As predicted by the World Economic Forum, within the next 16 years, management of chronic disease including neuroinflammation is predicted to cost the world a staggering $47 trillion in treatment and lost wages. The treatments currently available are rarely curative and have serious side effects. The use of plant-based substances for the treatment of various mental ailments has been prevalent for centuries [176]. Of these, flavonoids are an important group of more than 4000 polyphenolic compounds possessing a common phenylbenzopyrone structure (C6-C3-C6), which allows a wide range of biological activities [177,178]. Among other related flavonoids, apigenin, a naturally occurring plant flavone, is found in abundance in common fruits and vegetables such as parsley, tea, chamomile, wheat sprouts, and some seasonings. It represents about 0.8% of the total flavonoids consumed on a daily basis by the U.S. population, estimated by the department of food science and human nutrition [179]. For the successful development of any natural product lead compound as a therapeutic entity, several important characteristics such as increased bioavailability, long half-life, and slow absorption and excretion need to be fulfilled. Additionally, the bioactive molecule should be able to convene at the target organ at an effective concentration unaltered, for it to exert a suitable biological effect. The bioavailability of a number of flavonoids has been extensively studied and the results have shown rapid excretion and extensive metabolism following ingestion. Most of the flavonoids are detected in the blood stream within a few minutes to a few hours, with half-lives not exceeding 48 h, after which they are excreted from the body. However, apigenin shows comparatively slower absorption kinetics and delayed plasma clearance in pathogen-free Wistar rats after single oral administration [180]. Apigenin is seen in the blood stream only 24 h after ingestion, with a relatively longer half-life of 91.8 h. Apigenin is also recovered at basal levels up to 10 days post ingestion, indicating slow excretion kinetics due to slow decomposition in the liver. These remarkable pharmacokinetics warrant further studies in human subjects to ascertain bioactive concentrations of apigenin required to obtain the anti-inflammatory pharmacological effects that have been reported in vitro.
Apigenin is a low molecular weight flavone (molecular weight = 270.24kDa), practically insoluble in water, partially soluble in hot alcohols, and completely soluble in potassium hydroxide (KOH) and dimethylsulfoxide (DMSO). Apigenin is a major constituent of chamomile and when prepared as chamomile tea, it has been used for centuries as a natural remedy for relieving indigestion and gastritis. Chamomile preparations have also been traditionally used in skin care products for the treatment of cutaneous inflammation and other skin disorders [138]. All flavonoids are synthesized in plants via a common pathway known as the shikimic acid pathway, which converts carbohydrate precursors to aromatic amino acids. Apigenin is synthesized through this pathway from its precursor Naringenin by the action of flavone synthase. Further, apigenin derivatives are also produced by O- or C-glycosylation, methylation, and hydroxylation of apigenin (
Table 1)2).
I As mentioned earlier, it is relatively unclear whether naturally occurring structural modifications to the basic flavonoid enhances or suppresses its biological activities, especially its anti-inflammatory action. However, synthetic derivatives of apigenin have been reportedly generated to enhance various characteristics of the parent compound. The addition of different amines to the apigenin ring at the C-7 position generated two series of apigenin derivatives with enhanced anti-proliferative functions tested against different human cancer cell lines
[50][181]. Apigenin amino acid prodrugs formed through synthesizing several amino acid conjugates including di- and tri-peptide analogs may improve solubility, enhancing its biological activity and usage. Several groups have synthesized and tested amino acid derivatives to improve the oral bioavailability of the plant flavone, tricin. The tricin-alanine-glutamic acid conjugate (T-Ala-Glu) exhibited excellent bioavailability after oral administration
[51][182]. Similar derivatives can be generated with enhanced anti-inflammatory properties, as well as improving apigenin penetration across the BBB for its therapeutic potential in neuroinflammatory diseases.
Table 12. Naturally occurring apigenin derivatives.
| Name |
Structure |
Source |
Modification |
Biological Activity |
Reference |
| Apiin |
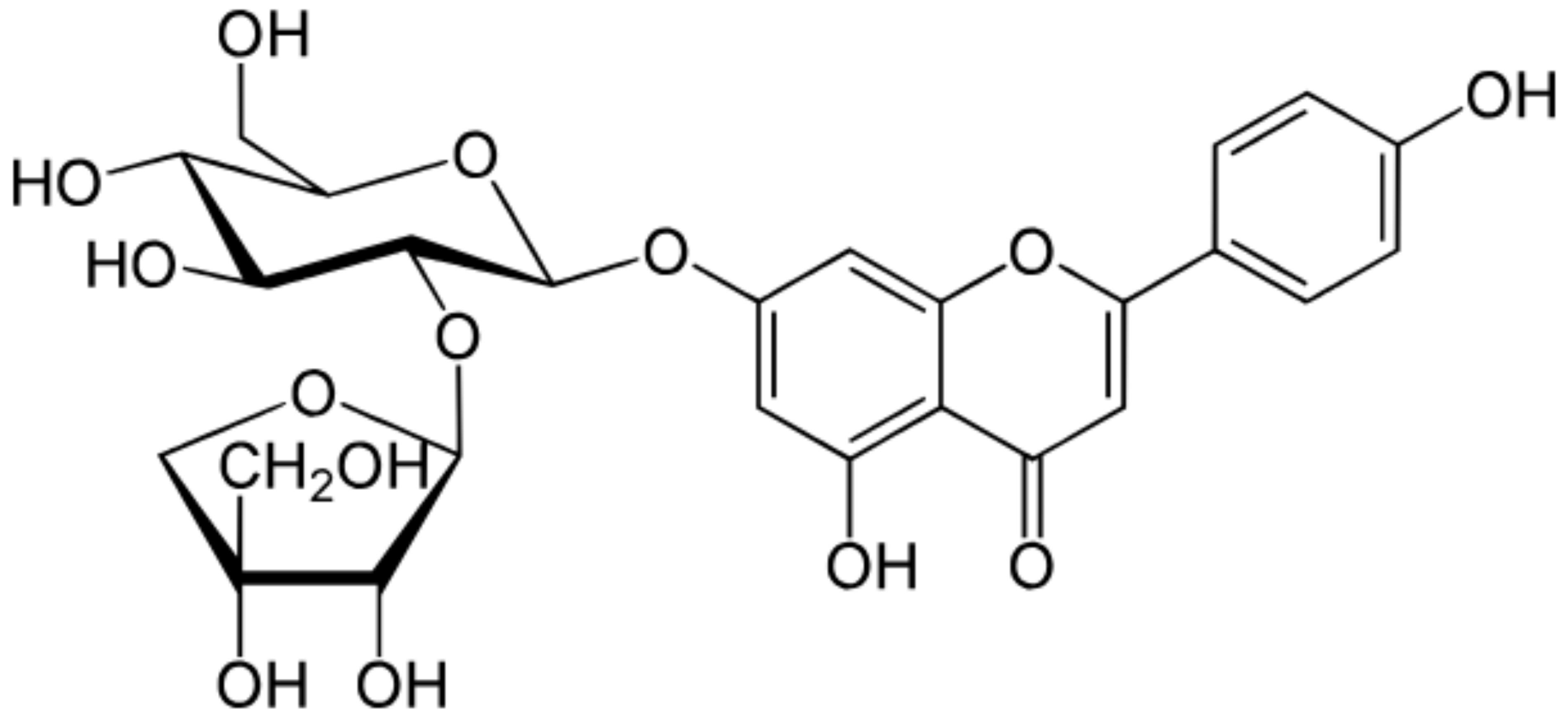 |
Parsley, Celery |
Glycosylation, Hydroxylation |
Anti-oxidant |
[52][53][54,55] |
| Apigetrin |
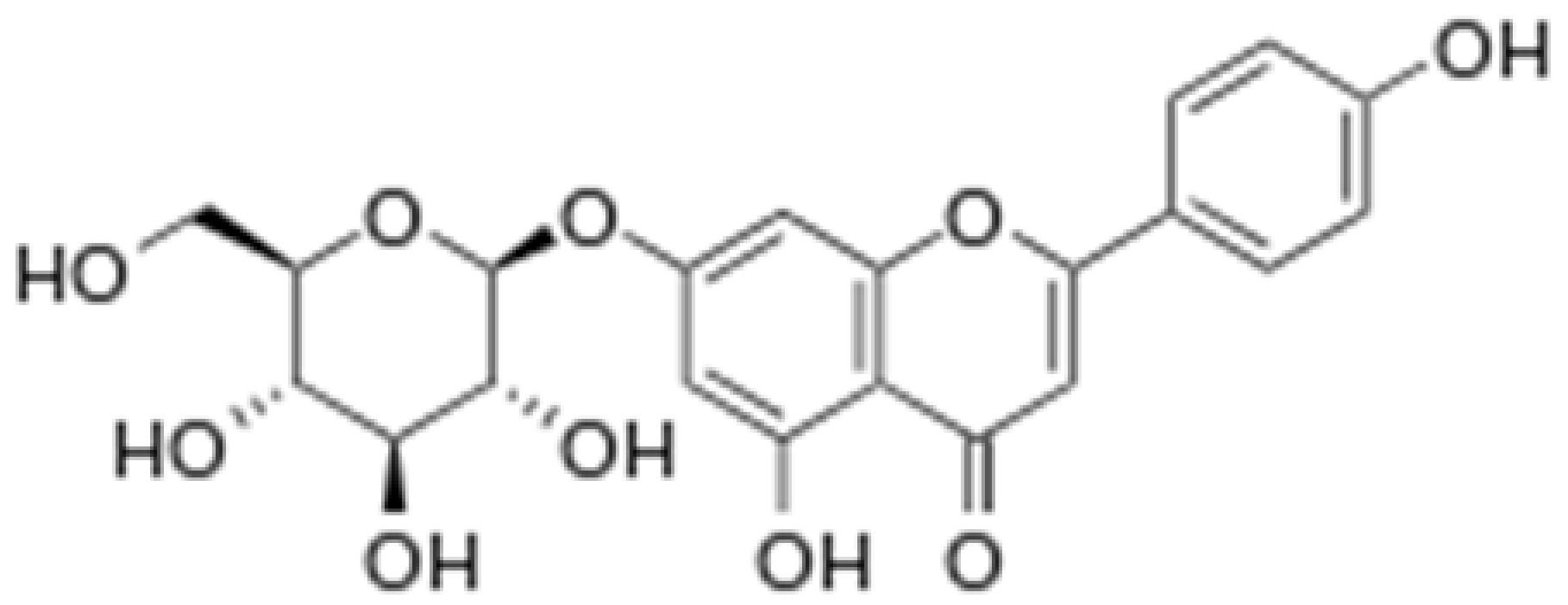 |
Roots of dandelion coffee |
Glycosylation |
Anti-inflammatory, anti-cancer |
[53][54][55,56] |
| Vitexin |
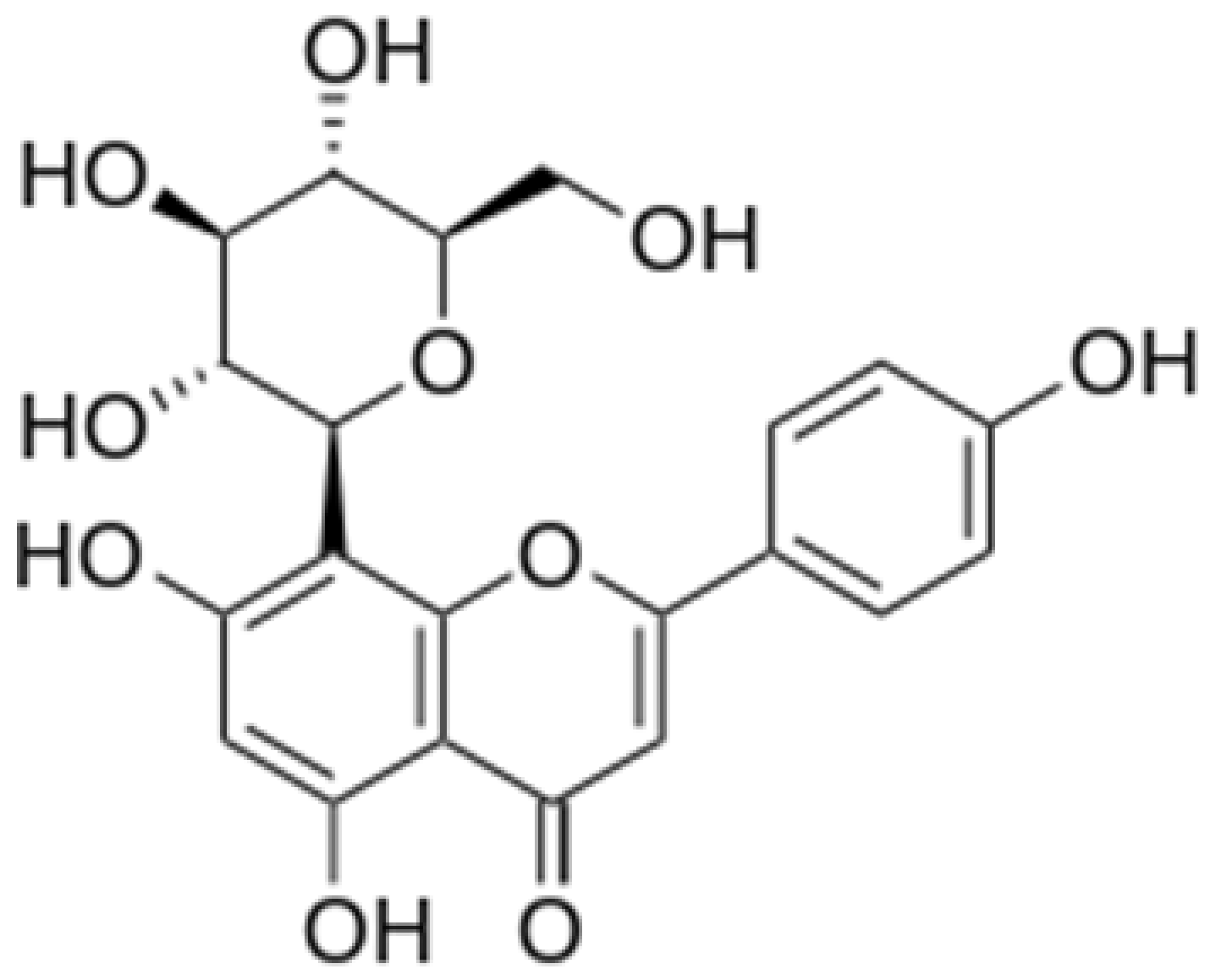 |
Mung bean, Bamboo leaves |
Glycosylation |
Anti-oxidant, neuroprotective, Anti-inflammatory |
[53][55][55,57] |
| Isovitexin |
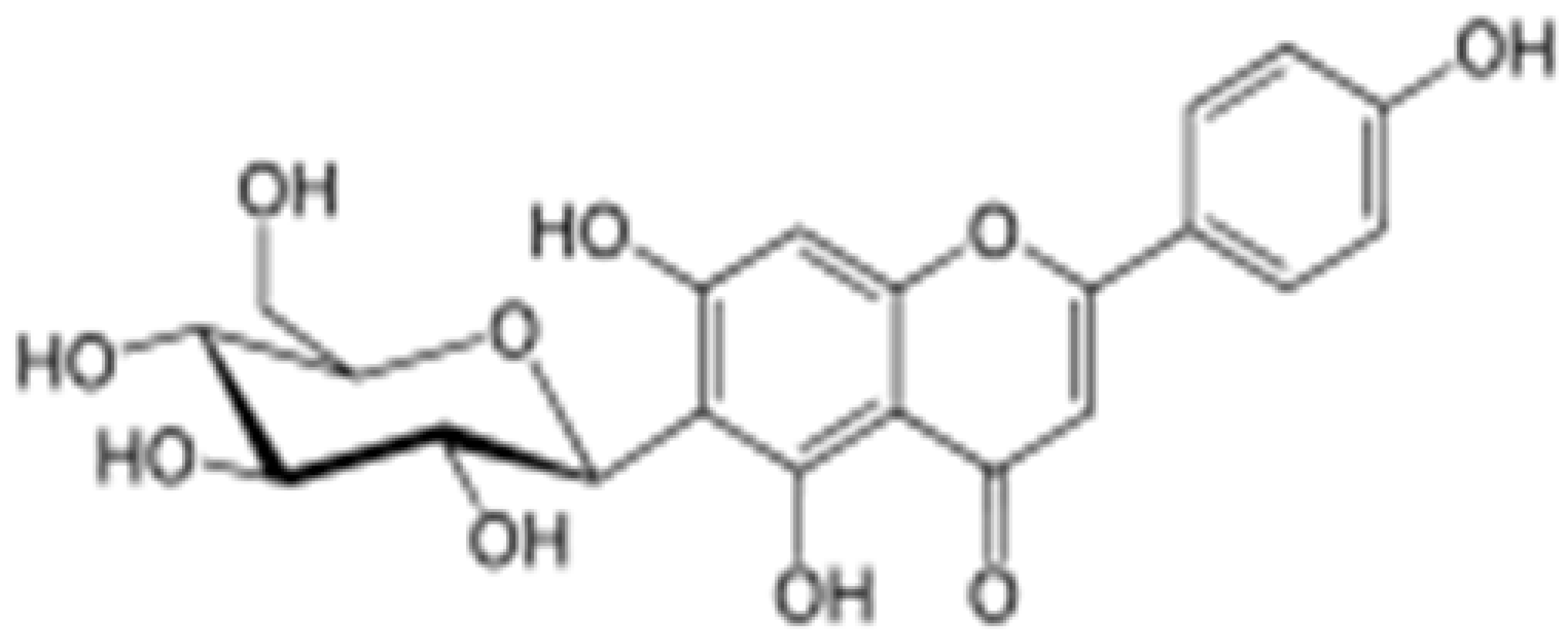 |
Mung bean, Ficus deltoidea |
Glycosylation, Hydroxylation |
Anti-inflammatory, anti-Alzheimer’s |
[53][55][55,57] |
| Rhoifolin |
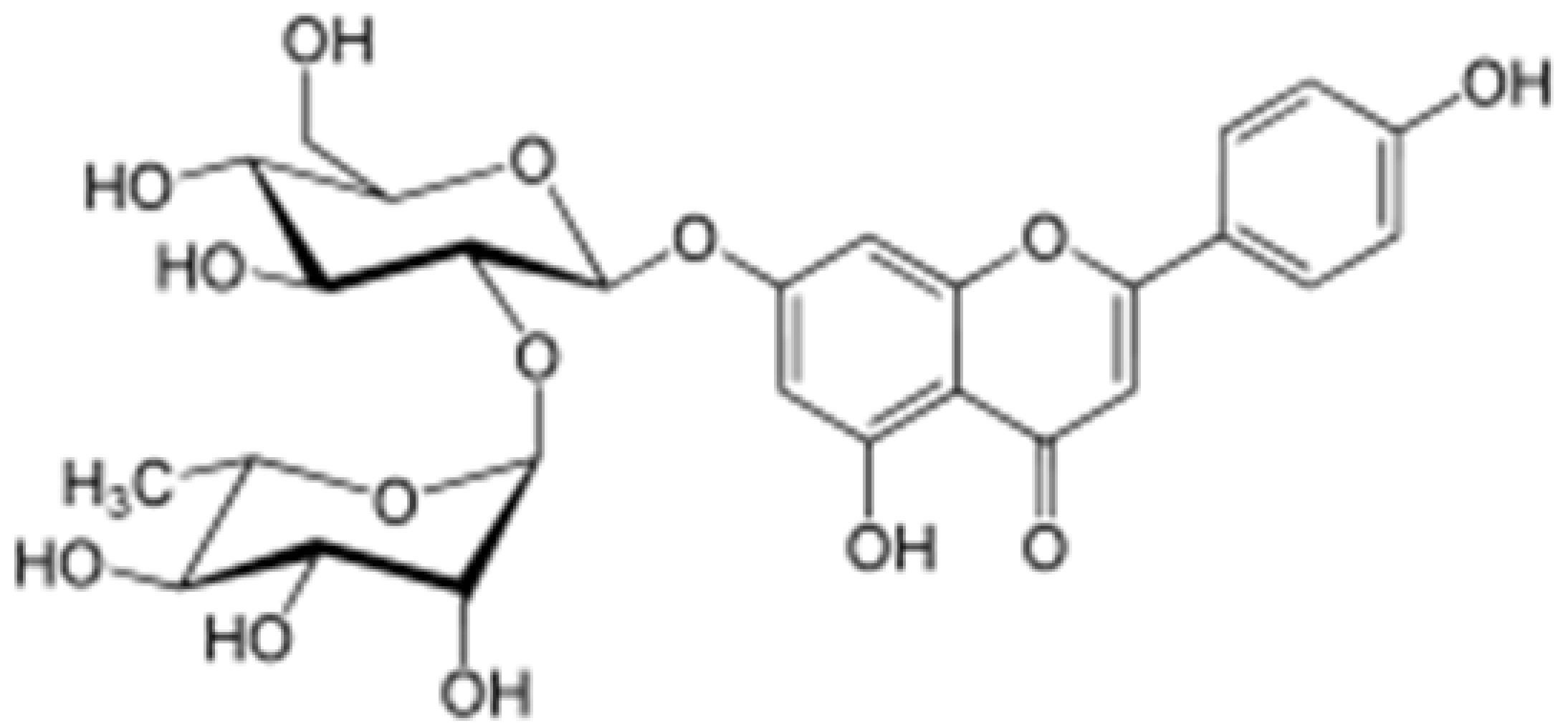 |
Orange,lupinus, Citrus grandis |
Hydroxylation |
Anti-microbial, anti-cancer, anti-inflammatory |
[53][56][55,58] |
| Schaftoside |
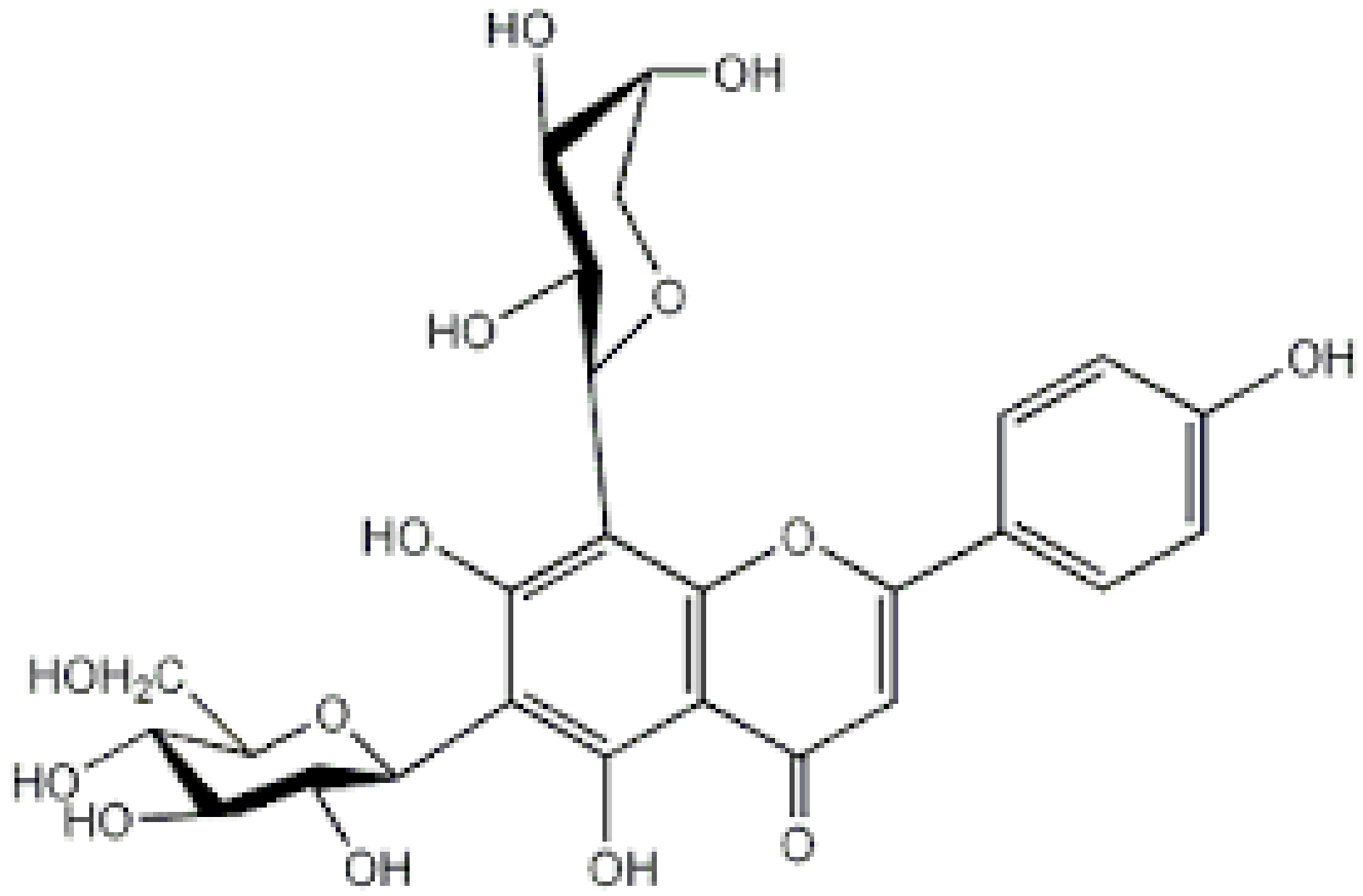 |
Arisaema heterophyllum |
Glycosylation |
Anti-melanogenic |
[53][57][55,59] |
| Acacetin |
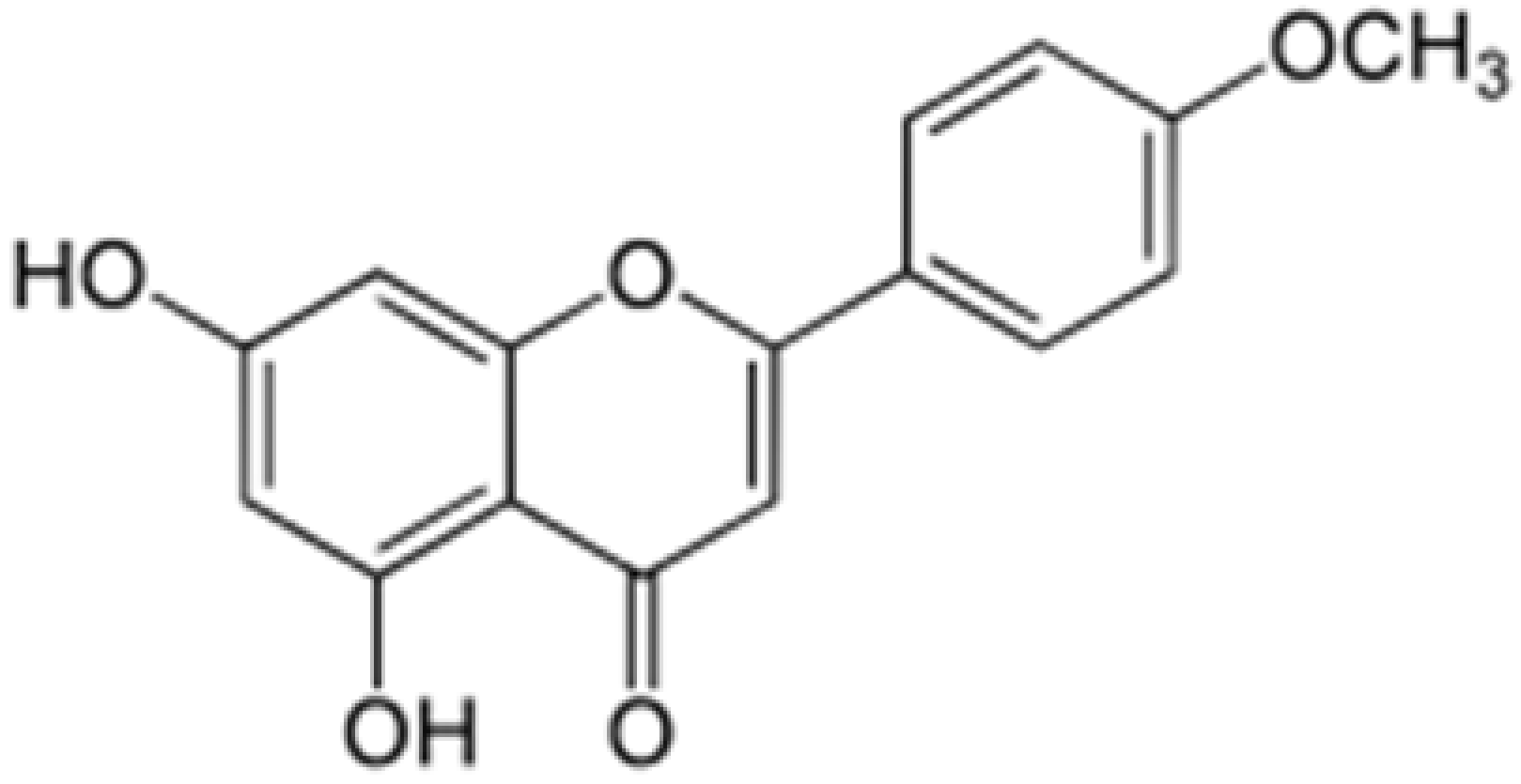 |
Turnera diffusa, Chrysanthemum morifolium |
Methylation |
Anti-inflammatory, antinociceptive |
[53][58][55,60] |
| Genkwanin |
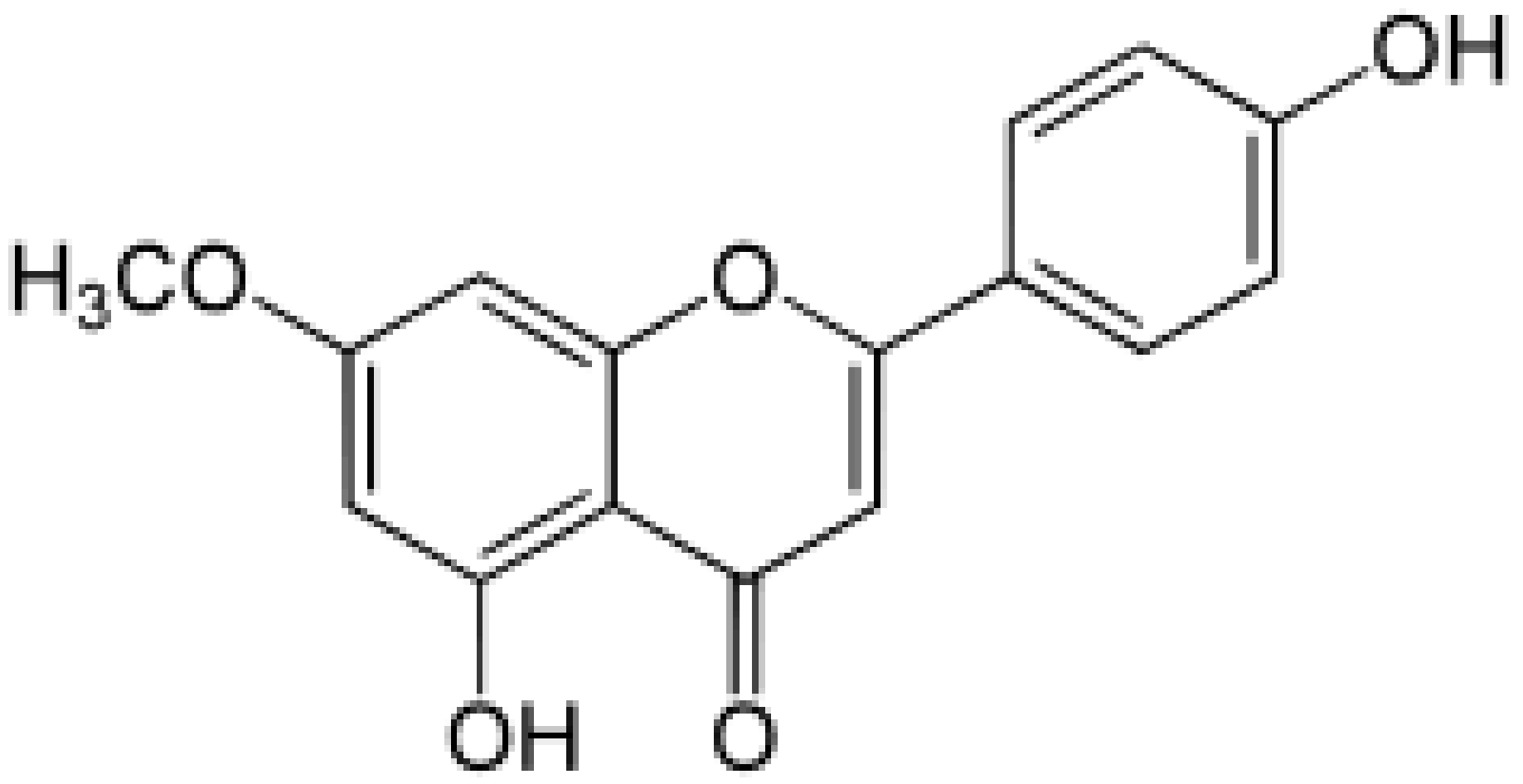 |
Genkwa flos, rosemary, seeds of Alnus glutinosa. |
Methylation |
Anti-tumor, anti-inflammatory |
5.2 Delivery Systems
Although the bioavailability and blood-brain barrier (BBB) penetration of apigenin is better than many other structurally related flavonoids, it may still not reach therapeutic levels to achieve the desired anti-inflammatory effects both in periphery and the CNS. To address this, seSeveral research groups have devised different delivery systems to increase apigenin bioavailability.
Nanosized drug delivery systems (NDDS) using liposome and polymer-based capsules
have been designed for the for biocompatible delivery of large quantities of apigenin
. These systems have been were designed and tested on
the hhuman macrovascular endothelial cell line EAhy926
, [183]. dThe
monstrating NDDS showed optimal drug loading and good stability over extended time periods, and
were non-toxic
ity to the
cells [59]EAhy926 cells. With extended release characteristics, these NDDS can serve as nanocarriers for apigenin delivery to targeted organs to curb localized inflammation. The daily intake of apigenin is estimated to vary from 3.4 to 26 mg/day across the different countries and its consumption is largely based on lifestyle choices. To achieve therapeutic effects, apigenin will need to be ingested in its purified form with optimal release kinetics.
Enteric polymer coated spheres were loaded with apigenin powder dispersed in an aqueous solution to allow targeted delivery to the intestine and colon, which are the main sites for absorption
[60][184]. These pellets were able to ensure apigenin release within 1 h and achieved therapeutic anti-oxidant effects, making it an optimal delivery system for enhanced apigenin uptake.
The BBB functions to not only allow the passage of essential nutrients and factors for the functioning of the brain, but also to limit the entry of therapeutic agents targeted against CNS abnormalities. This limits the number of drug entities that are able to cross the BBB to achieve the desired therapeutic effect. Though apigenin is a relatively small molecule that has been shown to cross the BBB, the intranasal delivery of apigenin, which bypasses the BBB and can gain entry in to the cerebrospinal fluid compartment via the olfactory pathway
[61][185], could be explored to achieve therapeutic dosages of apigenin delivered directly to the CNS.
An inhalable formulation of albumin nanoparticles loaded with apigenin has been constructed for
the del
ivery of apigenin to lung tissue
delivery [62][186]. Effective anti-oxidant properties against lung injury were reported with this delivery system, making it a viable inhalable drug formulation. However, further testing in an in vivo model
s of neuroinflammation
is necessary to assesswill provide useful information regarding CNS bioavailability and therapeutic reach.