Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Shusong Wu.
Cyanidin-3-glucoside (C3G) is a well-known natural anthocyanin and possesses antioxidant and anti-inflammatory properties. The catabolism of C3G in the gastrointestinal tract could produce bioactive phenolic metabolites, such as protocatechuic acid, phloroglucinaldehyde, vanillic acid, and ferulic acid, which enhance C3G bioavailability and contribute to both mucosal barrier and microbiota.
- cyanidin-3-glucoside
- phenolic metabolites
- gut microbiota
- signaling pathways
- intestinal injury
1. Introduction
Anthocyanins belong to polyphenols, which are one kind of secondary metabolite with polyphenolic structure widely occurring in plants. They serve as key antioxidants and pigments that contribute to the coloration of flowers and fruits. Although anthocyanins vary in different plants, six anthocyanidins, including pelargonidins, cyanidins, delphinidins, peonidins, petunidins, and malvidins, are considered as the major natural anthocyanidins. Berries, such as red raspberry (Rubus idaeus L.), blue honeysuckle (Lonicera caerulea L.), and mulberry are used as folk medicine traditionally, and their extracts have been used in the treatment of disorders such as cardiovascular disease [1], obesity [2], neurodegeneration [3], liver diseases [4], and cancer [5], in recent years. Cyanidin-3-glucoside (C3G) is one of the most common anthocyanins naturally found in black rice, black bean, purple potato, and many colorful berries. C3G possesses strong antioxidant activity potentially due to the two hydroxyls on the B ring [6], as shown in Figure 1. Recent studies have suggested that C3G potentially exerts functions primarily through C3G metabolites (C3G-Ms) [7], and more than 20 kinds of C3G-Ms have been identified in serum by a pharmacokinetics study in humans [8]. Although the function and mechanism of C3G-Ms are still not clear, protocatechuic acid (PCA) [9[9][10][11][12],10,11,12], phloroglucinaldehyde (PGA) [1], vanillic acid (VA) [13[13][14][15],14,15], ferulic acid (FA) [16,17[16][17][18][19],18,19], and their derivates represent the main bioactive metabolites of C3G due to their antioxidant and anti-inflammatory properties.
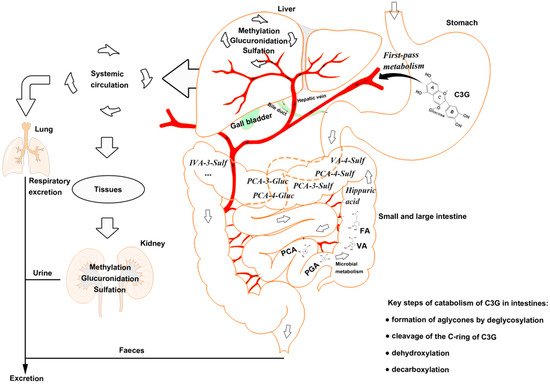
Figure 1. The catabolism process of cyanidin-3-glucoside (C3G) in an organism. C3G can be hydrolyzed to its aglycone by enzymes in the small intestine, and further degraded to phenolic compounds by gut microbiota. Microbial catabolism of C3G in the distal small intestine and large intestine is performed by the cleavage of the heterocyclic flavylium ring (C-ring), followed by dehydroxylation or decarboxylation to form multistage metabolites, which enter the liver and kidney by circulation. C3G, cyanidin-3-glucoside; FA, ferulic acid; PCA, protocatechuic acid; PGA, phloroglucinaldehyde; VA, vanillic acid.
2. Absorption and Catabolism of C3G in the Gastrointestine
Most of the anthocyanins remain stable in the stomach and upper intestine [20,21][20][21]. The stomach is considered as one of the predominant sites for anthocyanin and C3G absorption [22[22][23],23], although high concentration (85%) of anthocyanins has been found in the distal intestine [24]. There is potential for the first-pass metabolism of C3G in the stomach, that is, C3G can be effectively absorbed from the gastrointestinal tract and undergoes extensive first-pass metabolism, which can enter the systemic circulation as metabolites [25].
Anthocyanins are stable under acidic conditions but extremely unstable under alkaline conditions. The higher the pH is, the more colorless and substituent forms of anthocyanin are predominant [26]. The catabolism of C3G is mainly completed in the distal small intestine, such as ileum [22], and in the upper large intestine, such as the colon [27], with the decomposition by microbiota [28]. C3G can be hydrolyzed to their aglycones by enzymes in the small intestine, and further degraded to phenolic compounds by gut microbiota, in which microbial catabolism of C3G is performed by the cleavage of the heterocyclic flavylium ring (C-ring), followed by dehydroxylation or decarboxylation [29]. Subsequently, phase Ⅱ metabolites and multistage metabolites (including bacterial metabolites) can enter the liver and kidney to form more methylate, gluronide, and sulfate conjugated metabolites by enterohepatic circulation and blood circulation (Figure 1).
3. Biological Functions of C3G-Ms
Only several C3G-Ms have shown potential biological function, although more than 20 kinds of C3G-Ms have been identified [8,30][8][30]. PCA and phloroglucinaldehyde (PGA) are considered as the major bioactive phenolic metabolites produced by phase І metabolism, which undergo cleavage of the C ring of C3G. PCA can increase the antioxidant capacity of cells potentially by increasing the activity of antioxidant enzymes, such as catalase (CAT) in hypertensive rats or arthritis-model rats [31[31][32],32], superoxide dismutase (SOD) [33], and glutathione peroxidase (GPx) in mice or macrophages [33[33][34][35][36],34,35,36], and thus attenuate lipid peroxidation. Meanwhile, PCA has been reported to inhibit the production of inflammatory mediators, such as interleukin (IL)-6, tumor necrosis factor-α (TNF-α), IL-1β, and prostaglandin E2 (PGE2) [37,38,39][37][38][39], potentially by suppressing the activation of nuclear factor-κB (NF-κB) and extracellular signal-regulated kinase (ERK) [33,38][33][38] in murine BV2 microglia cells and colitis-model mice. PGA has also shown an inhibitory effect on inflammation potentially by modulating the production of IL-1β, IL-6, and IL-10 [40] in human whole blood cultures, although there are few reports about the molecular mechanisms. Our previous studies have revealed that both PCA and PGA are capable to down-regulate the MAPK pathway, especially suppress the activation of ERK, and PGA can directly bind to ERK1/2 [41] in murine macrophages.
Phase Ⅱ metabolites of C3G, such as PCA-3-glucuronide (PCA-3-Gluc), PCA-4-glucuronide (PCA-4-Gluc), PCA-3-sulfate (PCA-3-Sulf), PCA-4-sulfate (PCA-4-Sulf), VA, VA-4-sulfate (VA-4-Sulf), isovanillic acid (IVA), IVA-3-sulfate (IVA-3-Sulf), and FA, are mostly derived from PCA and PGA [1,8][1][8]. VA and FA represent the bioactive phenolic metabolites based on recent studies. VA may suppress the generation of reactive oxygen species (ROS) [42] and lipid peroxidation [32], potentially by increasing the activity of antioxidant enzymes such as SOD, CAT, and GPx [43[43][44],44], as well as the level of antioxidants such as vitamin E [43,44][43][44], vitamin C [43[43][44],44], and glutathione (GSH) [45] in mice, hamster, and diabetic hypertensive rats. Additionally, VA can inhibit the production of pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β, and IL-33 by down-regulating caspase-1 and NF-κB pathways [45,46,47][45][46][47] in mice or mouse peritoneal macrophages and mast cells. FA has also been reported to attenuate both oxidative stress and inflammation potentially by suppressing the production of free radicals (ROS and NO in rats, rat intestinal mucosal IEC-6 cell, or murine macrophages) [48[48][49][50],49,50], enhancing Nrf2 expression and down-stream antioxidant enzymes (SOD and CAT in rats or swiss albino mice) [48[48][51],51], and inhibiting the activation of proinflammatory proteins (p38 and IκB in HUVEC cells) [52] and cytokines production, such as IL-18 in HUVEC cells [52], IL-1β in mice [53], IL-6 in obese rats [54], and TNF-α in mice [53]. However, both VA and FA showed a limited effect on the activation of MAPK pathway and production of inflammatory cytokines, such as monocyte chemoattractant protein-1 (MCP-1) and TNF-α in a high-fat diet-induced mouse model of nonalcoholic fatty liver disease [41]. Table 1 summarizes the biological functions of the main bioactive metabolites, including PCA, PGA, VA, and FA.
Table 1. Biological functions of C3G-Ms.
| C3G-Ms | Biological Functions | Objects | Results |
|---|---|---|---|
| PCA | Antioxidant | Rats, mice, macrophages | Treatment with PCA increased T-AOC [31], catalase [33], SOD [33] and GPx [33,34,35,36][33][34][35][36] levels, but decreased ROS [35], MDA [31] and hydroperoxides [31] levels. |
| Anti-inflammatory | Mice, macrophages | PCA decreased IL-6 [33,37,39][33][37][39], TNF-α [33,39][33][39], IL-1β [33,39][33][39] and PGE2 production [39], and inhibited ERK, NF-κB p65 activation [33]. | |
| PGA | Anti-inflammatory | Mice, Human | PGA decreased serum levels of MCP-1 and TNF-α in high fat diet-induced mice [41]; PGA inhibited the production of IL-1β and IL-6 in human whole blood cultures after LPS stimulation, but no significant difference (p > 0.01) [40]. |
| VA | Antioxidant | Hamsters, mice, rats | VA increased SOD [43,44][43][44], catalase [43,44][43][44], GPx [43,44][43][44], vitamin E [43,44][43][44], vitamin C [43,44][43][44] and GSH [43,44,[44]45][43][45] levels. |
| Anti-inflammatory | Rats, mice, macrophages | VA inhibited caspase-1, NF-κB and MAPKs activation [45,46,47][45][46][47], decreased production of COX-2, PGE2 and NO [46], and reduced the levels of TNF-α [45,46][45][46], IL-6 [46,55][46][55], IL-1β [45] and IL-33 [45]. | |
| FA | Antioxidant | Rats, mice, IEC-6 cells | FA decreased the production of ROS [45,46,47][45][46][47], MDA [49], NO [49], enhanced SOD [48,49][48][49] and CAT [48,51][48][51] activity, and promoted the activation of Nrf2 [51]. |
| Anti-inflammatory | HUVEC cells, mice, rats | FA decreased the expression of caspase-1 [52], ICAM-1 [52], VCAM-1 [52], IL-18 [52], IL-1β [50,52,53,54][50][52][53][54], IL-6 [50,54][50][54], TNF-α [53], and inhibited the phosphorylation of p38 and IκB [52]. |
Notes: C3G-Ms, cyanidin-3-glucoside metabolites; CAT, catalase; COX-2, cyclooxygenase-2; ERK, extracellular signal-regulated kinase; FA, ferulic acid; GSH, glutathione; ICAM-1, intercellular adhesion molecule-1; LPS, lipopolysaccharide; MAPKs, mitogen-activated protein kinases; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde; NF-κB, nuclear factor-κB; NO, nitric oxide; PCA, protocatechuic acid; PGA, phloroglucinaldehyde; PGE2, prostaglandin E2; ROS, reactive oxygen species; T-AOC, total antioxidant capacity; VA, vanillic acid; VCAM-1, vascular cell adhesion molecule-1; SOD, superoxide dismutase; TNF-α, tumor necrosis factor-α.
References
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106.
- You, Y.; Yuan, X.; Liu, X.; Liang, C.; Meng, M.; Huang, Y.; Han, X.; Guo, J.; Guo, Y.; Ren, C.; et al. Cyanidin-3-glucoside increases whole body energy metabolism by upregulating brown adipose tissue mitochondrial function. Mol. Nutr. Food Res. 2017, 61, 1700261.
- Tremblay, F.; Waterhouse, J.; Nason, J.; Kalt, W. Prophylactic neuroprotection by blueberry-enriched diet in a rat model of light-induced retinopathy. J. Nutr. Biochem. 2013, 24, 647–655.
- Wu, S.; Yano, S.; Hisanaga, A.; He, X.; He, J.; Sakao, K.; Hou, D.-X. Polyphenols from Lonicera caerulea L. berry attenuate experimental nonalcoholic steatohepatitis by inhibiting proinflammatory cytokines productions and lipid peroxidation. Mol. Nutr. Food Res. 2017, 61, 1600858.
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-alpha and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58.
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159.
- Bharat, D.; Ramos, R.; Cavalcanti, M.; Petersen, C.; Begaye, N.; Cutler, B.R.; Costa, M.M.A.; Ramos, R.K.L.G.; Ferreira, M.R.; Li, Y.; et al. Blueberry metabolites attenuate lipotoxicity-induced endothelial dysfunction. Mol. Nutr. Food Res. 2018, 62, 1700601.
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282.
- Ma, Y.; Chen, F.; Yang, S.; Chen, B.; Shi, J. Protocatechuic acid ameliorates high glucose-induced extracellular matrix accumulation in diabetic nephropathy. Biomed. Pharmacother. 2018, 98, 18–22.
- Jang, S.-A.; Song, H.S.; Kwon, J.E.; Baek, H.J.; Koo, H.J.; Sohn, E.-H.; Lee, S.R.; Kang, S.C. Protocatechuic acid attenuates trabecular bone loss in ovariectomized mice. Oxidative Med. Cell. Longev. 2018, 2018, 7280342.
- Molehin, O.R.; Adeyanju, A.A.; Adefegha, S.A.; Akomolafe, S.F. Protocatechuic acid mitigates adriamycin-induced reproductive toxicities and hepatocellular damage in rats. Comp. Clin. Pathol. 2018, 27, 1681–1689.
- Jang, S.-E.; Choi, J.-R.; Han, M.J.; Kim, D.-H. The preventive and curative effect of cyanidin-3β-D-glycoside and its metabolite protocatechuic acid against TNBS-induced colitis in mice. Nat. Prod. Sci. 2016, 22, 282–286.
- Bhavani, P.; Subramanian, P.; Kanimozhi, S. Preventive efficacy of vanillic acid on regulation of redox homeostasis, matrix metalloproteinases and cyclin D1 in rats bearing endometrial carcinoma. Indian J. Clin. Biochem. 2017, 32, 429–436.
- Rasheeda, K.; Bharathy, H.; Fathima, N.N. Vanillic acid and syringic acid: Exceptionally robust aromatic moieties for inhibiting in vitro self-assembly of type I collagen. Int. J. Biol. Macromol. 2018, 113, 952–960.
- Khoshnam, S.E.; Farbood, Y.; Moghaddam, H.F.; Sarkaki, A.; Badavi, M.; Khorsandi, L. Vanillic acid attenuates cerebral hyperemia, blood-brain barrier disruption and anxiety-like behaviors in rats following transient bilateral common carotid occlusion and reperfusion. Metab. Brain Dis. 2018, 33, 785–793.
- Tanihara, F.; Hirata, M.; Nhien, N.T.; Hirano, T.; Kunihara, T.; Otoi, T. Effect of ferulic acid supplementation on the developmental competence of porcine embryos during in vitro maturation. J. Vet. Med Sci. 2018, 80, 1007–1011.
- Peresa, D.D.A.; Sarrufb, F.D.; de Oliveirac, C.A.; Velascoa, M.V.R.; Babya, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B Biol. 2018, 185, 46–49.
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2018, 10, 713.
- Zhang, S.; Wang, P.; Zhao, P.; Wang, D.; Zhang, Y.; Wang, J.; Chen, L.; Guo, W.; Gao, H.; Jiao, Y. Pretreatment of ferulic acid attenuates inflammation and oxidative stress in a rat model of lipopolysaccharide-induced acute respiratory distress syndrome. Int. J. Immunopathol. Pharmacol. 2018, 32, 394632017750518.
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180.
- Yang, P.; Yuan, C.; Wang, H.; Han, F.; Liu, Y.; Wang, L.; Liu, Y. Stability of Anthocyanins and Their Degradation Products from Cabernet Sauvignon Red Wine under Gastrointestinal pH and Temperature Conditions. Molecules 2018, 23, 354.
- Cai, H.; Thomasset, S.C.; Berry, D.P.; Garcea, G.; Brown, K.; Stewarda, W.P.; Gescher, A.J. Determination of anthocyanins in the urine of patients with colorectal liver metastases after administration of bilberry extract. Biomed. Chromatogr. 2011, 25, 660–663.
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.-L.; Remesy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182.
- Moraisa, C.A.; de Rossoa, V.V.; Estadellaa, D.; Pisani, L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016, 33, 1–7.
- Jim, F. Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: Extensive presystemic metabolism reduces apparent bioavailability. J. Agric. Food Chem. 2014, 62, 3904–3911.
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871.
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142.
- Hanske, L.; Engst, W.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013, 109, 1433–1441.
- Zhang, X.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B. An exploratory study of red raspberry (Rubus idaeus L.) (poly)phenols/metabolites in human biological samples. Food Funct. 2018, 9, 806–818.
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048.
- Safaeiana, L.; Emamia, R.; Hajhashemia, V.; Haghighatian, Z. Antihypertensive and antioxidant effects of protocatechuic acid in deoxycorticosterone acetate-salt hypertensive rats. Biomed. Pharmacother. 2018, 100, 147–155.
- Lende, A.B.; Kshirsagar, A.D.; Deshpande, A.D.; Muley, M.M.; Patil, R.R.; Bafna, P.A.; Naik, S.R. Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacology 2011, 19, 255–263.
- Crespo, I.; San-Miguel, B.; Mauriz, J.L.; Ortiz de Urbina, J.; Almar, M.; Tuñón, M.J.; González-Gallego, J. Protective effect of protocatechuic acid on TNBS-induced colitis in mice is associated with modulation of the SphK/S1P signaling pathway. Nutrients 2017, 9, 288.
- Ma, L.; Wang, G.; Chen, Z.; Li, Z.; Yao, J.; Zhao, H.; Wang, S.; Ma, Z.; Chang, H.; Tian, X. Modulating the p66shc signaling pathway with protocatechuic acid protects the intestine from ischemia-reperfusion injury and alleviates secondary liver damage. Sci. World J. 2014, 2014, 1–11.
- Varì, R.; Scazzocchio, B.; Santangelo, C.; Filesi, C.; Galvano, F.; D’Archivio, M.; Masella, R.; Giovannini, C. Protocatechuic acid prevents oxLDL-induced apoptosis by activating JNK/Nrf2 survival signals in macrophages. Oxid. Med. Cell. Longev. 2015, 2015, 1–11.
- Cheng, Y.T.; Lin, J.A.; Jhang, J.J.; Yen, G.C. Protocatechuic acid-mediated DJ-1/PARK7 activation followed by PI3K/mTOR signaling pathway activation as a novel mechanism for protection against ketoprofen-induced oxidative damage in the gastrointestinal mucosa. Free Radic. Biol. Med. 2019, 130, 35–47.
- Amini, A.M.; Spencer, J.P.E.; Yaqoob, P. Effects of pelargonidin-3-O-glucoside and its metabolites on lipopolysaccharide-stimulated cytokine production by THP-1 monocytes and macrophages. Cytokine 2018, 103, 29–33.
- Wang, H.-Y.; Wang, H.; Wang, J.-H.; Wang, Q.; Ma, Q.-F.; Chen, Y.-Y. Protocatechuic acid inhibits inflammatory responses in LPS-stimulated BV2 Microglia via NF-kappaB and MAPKs signaling pathways. Neurochem. Res. 2015, 40, 1655–1660.
- Lin, C.-Y.; Huang, C.-S.; Huang, C.-Y.; Yin, M.-C. Anticoagulatory, antiinflammatory, and antioxidative effects of protocatechuic acid in diabetic mice. J. Agric. Food Chem. 2009, 57, 6661–6667.
- Amini, A.M.; Muzs, K.; Spencer, J.P.; Yaqoob, P. Pelargonidin-3-O-glucoside and its metabolites have modest anti-inflammatory effects in human whole blood cultures. Nutr. Res. 2017, 46, 88–95.
- Wu, S.; Hu, R.; Tan, J.; He, Z.; Liu, M.; Li, Y.; He, X.; Hou, D.-X.; Luo, J.; He, J. Abstract WP534: Cyanidin 3-glucoside and its Metabolites Protect Against Nonalcoholic Fatty Liver Disease: Crosstalk Between Serum Lipids, Inflammatory Cytokines and MAPK/ERK Pathway. Stroke 2019, 50 (Suppl. 1), AWP534.
- Amin, F.U.; Shah, S.A.; Kim, M.O. Vanillic acid attenuates Abeta1-42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753.
- Anbalagan, V.; Raju, K.; Shanmugam, M. Assessment of lipid peroxidation and antioxidant status in vanillic acid treated 7,12-dimethylbenzaanthracene induced hamster buccal pouch carcinogenesis. J. Clin. Diagn. Res. 2017, 11, BF01–BF04.
- Vinothiya, K.; Ashokkumar, N. Modulatory effect of vanillic acid on antioxidant status in high fat diet-induced changes in diabetic hypertensive rats. Biomed. Pharmacother. 2017, 87, 640–652.
- Calixto-Campos, C.; Carvalho, T.T.; Hohmann, M.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R.; et al. Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFkB activation in mice. J. Nat. Prod. 2015, 78, 1799–1808.
- Kim, M.-C.; Kim, S.-J.; Kim, D.-S.; Jeon, Y.-D.; Park, S.J.; Lee, H.S.; Um, J.-Y.; Hong, S.-H. Vanillic acid inhibits inflammatory mediators by suppressing NF-kappaB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532.
- Jeong, H.-J.; Nam, S.-Y.; Kim, H.-Y.; Jin, M.H.; Kim, M.H.; Roh, S.S.; Kim, H.-M. Anti-allergic inflammatory effect of vanillic acid through regulating thymic stromal lymphopoietin secretion from activated mast cells. Nat. Prod. Res. 2018, 32, 2945–2949.
- Ghosh, S.; Chowdhury, S.; Sarkar, P.; Sil, P.C. Ameliorative role of ferulic acid against diabetes associated oxidative stress induced spleen damage. Food Chem. Toxicol. 2018, 118, 272–286.
- He, S.; Guo, Y.; Zhao, J.; Xu, X.; Song, J.; Wang, N.; Liu, Q. Ferulic acid protects against heat stress-induced intestinal epithelial barrier dysfunction in IEC-6 cells via the PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Int. J. Hyperth. 2018, 35, 112–121.
- Szulc-Kielbik, I.; Kielbik, M.; Klink, M. Ferulic acid but not alpha-lipoic acid effectively protects THP-1-derived macrophages from oxidant and pro-inflammatory response to LPS. Immunopharmacol. Immunotoxicol. 2017, 39, 330–337.
- Das, U.; Manna, K.; Khan, A.; Sinha, M.; Biswas, S.; Sengupta, A.; Chakraborty, A.; Dey, S. Ferulic acid (FA) abrogates gamma-radiation induced oxidative stress and DNA damage by up-regulating nuclear translocation of Nrf2 and activation of NHEJ pathway. Free Radic. Res. 2017, 51, 47–63.
- Liu, J.-L.; He, Y.-L.; Wang, S.; He, Y.; Wang, W.-Y.; Li, Q.-J.; Cao, X.-Y. Ferulic acid inhibits advanced glycation end products (AGEs) formation and mitigates the AGEs-induced inflammatory response in HUVEC cells. J. Funct. Foods 2018, 48, 19–26.
- Zhou, Q.; Gong, X.; Kuang, G.; Jiang, R.; Xie, T.; Tie, H.; Chen, X.; Li, K.; Wan, J.; Wang, B. Ferulic acid protected from kidney ischemia reperfusion injury in mice: Possible mechanism through increasing adenosine generation via HIF-1alpha. Inflammation 2018, 41, 2068–2078.
- Salazar-López, N.J.; Astiazarán-García, H.; González-Aguilar, G.A.; Loarca-Piña, G.; Ezquerra-Brauer, J.M.; Domínguez Avila, J.A.; Robles-Sánchez, M. Ferulic acid on glucose dysregulation, dyslipidemia, and inflammation in diet-induced obese rats: An integrated study. Nutrients 2017, 9, 675.
- Kim, S.-J.; Kim, M.-C.; Um, J.-Y.; Hong, S.-H. The beneficial effect of vanillic acid on ulcerative colitis. Molecules 2010, 15, 7208–7217.
More
