Yeast communities associated with insects were identified either from entire insect bodies, which were previously surface-sterilized or not, or from dissected organs using culture-dependent and independent approaches. Independent cultural approaches usually involved DNA extractions from insect tissues followed by the amplification of taxonomic markers allowing a discrimination at the genus or species level, such as the Internal Transcribed Spacer (ITS) regions and the D1/D2 region of 26S ribosomal DNA.
- insect-microbiota interactions
- mycobiota
- yeast communities
- insects
1. Introduction
With nearly one million described species and 5.5 million estimated ones, insects represent more than 80% of the animal biodiversity on Earth [1]. Such diversity is reflected by a broad spectrum of evolutionary acquired traits, some of them being linked to their feeding mode [2]. The evolutionary success of many insects is closely tied to symbiotic associations with microorganisms having complementary potential that is otherwise lacking in insects and restricts them when inhabiting an ecologically challenging niche or invading new environments [3][4][3,4]. Therefore, our understanding of insect biology is facing a paradigm shift where these higher organisms can no longer be considered as an isolated entity and instead should be studied in relation with its microbiota (bacteria, fungi, protists, and viruses) with which it interacts and forms a metaorganism, often referred to as the holobiont [5][6][7][8][5,6,7,8].
To date, most studies have mainly focused on bacteria which establish parasitic, commensal, or symbiotic relationships with their hosts by colonizing different tissues such as ovaries [9], cuticle [10], or specialized host cells (bacteriocytes) often grouped into an organ called the bacteriome [11]. However, most of bacterial microbiota inhabit the digestive tract [3][4][3,4], which is composed of three regions with specific functions ( Figure 1 ). These regions vary extensively in terms of morphology and physicochemical properties across insect orders, factors that are known to greatly influence microbial community structure [3]. The midgut, which hosts a dense and diverse microbial community in most insect orders, is the primary site of digestion and absorption [4]. In comparison, few studies to date have investigated the bacterial diversity in the foregut (the region dedicated to food intake, storage, filtering and partial digestion). In Diptera (including flies and mosquitoes) and Lepidoptera (butterflies and moths), the crop is a ventral diverticulum of the oesophagus that serves as primary storage organ for sugars from the nectar before it is transferred into the midgut for digestion [2]. Interestingly, a diverse and rich bacterial community was recently observed in the crop of mosquitoes, raising questions about symbiotic associations occurring in this organ [12][13][12,13]. Finally, in the hindgut where the bacterial density is very low for certain insect orders and stronger for others ( Figure 1 ), the absorption is completed and feces are formed.
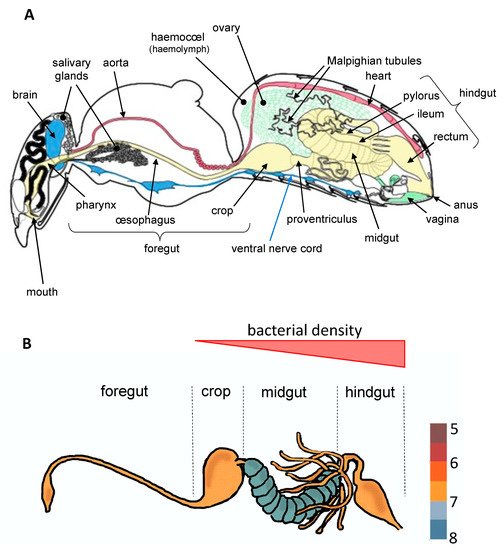
Insect bacterial microbiota offer a wide range of benefits to their host, ranging from increased fecundity [15], oviposition [16], and longevity [17] to shorter larval development [18]. Associated bacteria also influence many other aspects of insect biology, such as complementing host nutrition [19], facilitating dietary breakdown [20], providing protection against pathogens [21][22][21,22], and performing the detoxification of xenobiotics or dietary components [23][24][25][26][23,24,25,26]. The nature of gut microbiota-host associations appears to be variable among insects. While weevils [27], burying beetles [28], and social insects such as termites [29][30][29,30], bees [31], or certain ants [32] harbor specialized gut microbial communities mostly transmitted vertically and representing longstanding microbiota-host interactions, other insects like fruit flies or mosquitoes are mainly colonized by transient microbial communities acquired from the environment [33][34][33,34].
While an increasing number of studies on insect-associated microbiota have focused on bacteria, other microbial partners such as fungi have been more neglected [35]. Fungal communities (mycobiota) and more particularly yeasts have been demonstrated to be associated with many insect species [36]. Yeasts, which can dominate the mycobiota of certain insects, establish mostly commensal or symbiotic relationships with their host. Like bacteria, yeasts colonize different tissues, such as cuticle, and some yeast species referred to as yeast-like symbionts (YLS) or endosymbionts are localized in fat body specialized cells (mycetocytes) of certain insect species belonging to the Hemiptera and Coleoptera orders [36]. However, yeasts predominantly colonize the digestive tract where they may act as nutrient providers, digestion facilitators, or protectors against pathogens and toxic compounds [37]. Insects are then highly dependent on their gut microbiota, including yeasts, for their development and survival. Based on the degree of dependence, their association can be classified as obligate (or primary) and facultative (or secondary). If YLS located in the mycetocytes of the planthopper Nilaparvata lugens [38] and the aphid Cerataphis brasiliensis [39] are primary symbionts, some endosymbiotic yeasts are considered secondary symbionts, as they are associated with bacterial species. For example, Metschnikowia pimensis and another unidentified YLS (Hp-YSL) of the planthopper Hishimonus phycitis are associated with six bacterial endosymbionts including Sulcia and Nasuia species [40]. Similarly, in several cicada species ( Meimuna opalifera , Graptopsaltria nigrofuscata , Cryptotympana facialis , Hyalessa maculaticollis , and Mogannia minuta ), the primary bacterial endosymbionts Sulcia is associated with an YLS phylogenetically related to entomoparasitic Ophiocordyceps fungi [41]. This review highlights the diversity of commensal and symbiotic yeast communities associated with insects, as well as their impact on insect life-history traits (development, survival, reproduction), immunity, and behavior. As Drosophila melanogaster -yeast interactions have been extensively documented [42][43][42,43], this insect species was not included in the present review.
2. Diversity of Yeast Communities Associated with Insects and Variation Factors
The diversity of yeast communities was mostly studied for insect species with a major impact on humans and their environment such as crop auxiliaries (lacewings) [44][45][44,45], pollinators (bees, bumblebees, fruit flies, or floricolous beetles) [46][47][48][49][46,47,48,49], plant pests (moths, planthoppers, bark beetles) [6][50][51][52][6,50,51,52] and pathogen vectors (mosquitoes, sandflies) [53][54][55][53,54,55]. Yeast communities associated with insects were identified either from entire insect bodies, which were previously surface-sterilized [51][55][51,55] or not [48][49][48,49], or from dissected organs [13][50][56][13,50,56] using culture-dependent [49][57][58][49,57,58] and independent approaches [59][60][59,60]. Independent cultural approaches usually involved DNA extractions from insect tissues followed by the amplification of taxonomic markers allowing a discrimination at the genus or species level, such as the Internal Transcribed Spacer (ITS) regions and the D1/D2 region of 26S ribosomal DNA. Amplified sequences analyzed using DGGE [38][50][38,50], T-RFLP [61], Sanger [62][63][62,63], or high-throughput sequencing [55][64][55,64] were used to characterize insect associated-yeast communities.
Depending on the insect order, the composition of associated-yeast communities was not equally analyzed for all developmental stages ( Table S1 ). While only larvae were studied for Lepidoptera [50][65][50,65], the adult stage was preferentially analyzed for many other insect orders [41][51][55][66][67][68][41,51,55,66,67,68]. However, for some species belonging to several insect groups, such as mosquitoes [53], bark or sap beetles [6][69][6,69], and planthoppers [64], all life stages were analyzed and the presence of yeast species was detected at all developmental stages ( Table S1 ). These insect-yeast communities are mainly acquired from the environment [68][70][71][72][73][68,70,71,72,73]. For example, mosquito larvae acquire yeast communities mainly from the water of breeding sites, while adults obtain it from water at emergence as well as from sugar (plants or flower nectars) and/or blood meals for females during their entire life span [74]. In Hymenoptera (bees and bumblebees), adults acquire yeasts mainly from the nectar of flowers, while larvae obtain them from the provisions (pollen) supplied by adults [63][75][63,75].
As previously mentioned, insects acquire a large part of their yeast communities from their nutrient sources (flowers, fruits, sap, etc.) and/or breeding sites [47][53][58][68][71][76][47,53,58,68,71,92]. The environment is therefore one of the main factors shaping yeast communities associated with insects. A study analyzing the structure of yeast communities associated with several Drosophila species worldwide has shown that the insect diet has a greater impact than the host species per se [46]. Similarly, Lachance et al. [77][84] demonstrated that the composition and structure of yeast communities inhabiting the ventral diverticulum of Drosophila species feeding on cactus sap ( Drosophila mojavensis , D. mettleri …) are very different from those feeding on sap or tree fruits ( D. pseudoobscura , D. Miranda …). Yeasts vectored by stingless bees differ in southeastern and northern Neotropical savannas of Brazil, suggesting a strong influence of the visited vegetation [49]. Yeast communities associated with bark and ambrosia beetles were demonstrated to be strongly influenced by environmental factors such as host tree species and seasons [68][73][78][68,73,93].
The sex and social status of insects may also have a significant impact on the structure of yeast communities. In the planthoppers N. lugens [79][77] and D. kuscheli [80][76], YLS abundance gradually increases until the adult stage and remains relatively stable in females, while it strongly decreases upon emergence in males. In Ae. albopictus , yeasts belonging to the genus Aureobasidium are 11 to 15 times more abundant in the ventral diverticulum and midgut of males compared to females [13]. Yeast community composition is also affected by the social status of their hosts, as has been demonstrated for Apis mellifera bees. The gut of young bees and nurses presents a low yeast diversity and is highly dominated by Saccharomyces species (representing 97% to 99% of the yeast diversity). In contrast, foraging bees and queens are colonized by diverse yeast species and dominated by Zygosaccharomyces species (87%), respectively [81][86].
3. Influence of Yeasts on Insect Life-History Traits and Immune System
Whatever their stage of development, insects may use obligate or facultative yeast symbionts to compensate diverse metabolic functions. Yeasts associated with insects are known to facilitate the host feeding on recalcitrant food [82][83][84][82,89,101], provide immunity and protection against various pathogens and parasites [47][85][47,102], mediate inter- and intra-specific communication diet [86][87][103,104], aid digestion, and supply essential amino acids, metabolic compounds, and nutrients [39][88][89][39,78,105]. Those yeasts are essential for the optimal development and survival of many insects, demonstrated by the fact that Drosophila suzukii larvae reared in a yeast-free environment do not reach the pupal stage [90][91][106,107]. It has also been demonstrated that axenic mosquito larvae (microbiota-free larvae) exhibit delays in growth of more than six days [18] compared to conventionally-raised ones, or do not develop beyond the first instar, while the development is restored when living yeasts are supplied [92][108]. Similarly, in the brown planthopper ( N. lugens ), the absence of yeast-like symbionts in mycetocytes prevents the abdominal segmentation and the differentiation of the embryo [93][109], while a decrease in their density leads to a reduction in nymph weight [94][110].
At the adult stage, several phytophagous and blood-sucking insects feed on plant substances enriched in fructose, glucose, and sucrose [95][96][97][111,112,116]. If a certain proportion of these plant sugars is digested by enzymes contained in saliva and directly assimilated by the insect, most of them are stored in the crop or in the ventral diverticulum where a wide variety of yeast genera are present, such as Candida , Debaryomyces , Hanseniaspora , Meyerozyma , Metschnikowia , and Pichia ( Table S1 ) [12][13][47][77][98][12,13,47,84,119]. Sugars will then be gradually transported to the midgut where they will preferably be used as an energy source by the microbiota, and particularly yeasts [99][87]. For example, it has been shown that yeasts of the genus Malassezia associated with both male and female Ae. albopictus actively utilize fructose, while yeasts of the genus Cyberlindnera are more active in females [99][87].
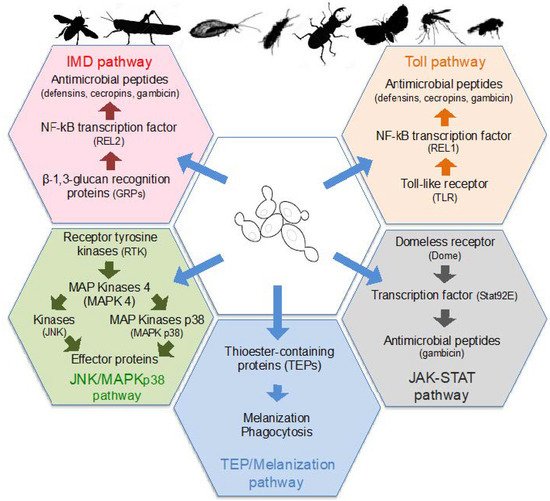
Insects only have an innate immune system that is based on the recognition of conserved microbe-associated molecular patterns (MAMPs) by a set of pattern-recognition receptors (PRRs) localized on the surface of host cells [100][127]. Several classes of PRRs are able to detect fungal surface molecules and secondary metabolites, which then induce the activation of protein kinases or transcription factors. In turn, those protein kinases and transcription factors stimulate the production of insect antimicrobial peptides (AMPs) including cecropins, defensins, diptericin, and gambicin, or other effector molecules, as well as phagocytic and melanization responses ( Figure 2 ). Infection by fungi, and therefore yeasts, activate several signaling pathways, and more particularly the Toll and TEP/Melanization pathways [100][101][127,128].
Other mechanisms, such as resource competition or production of antimicrobial compounds (toxins or other), allow yeasts to inhibit colonization of the insect host by entomopathogens or human pathogens. An in vitro study has demonstrated that yeasts of the species M. reukaufii , S. bombi , W. bombiphila , previously isolated from the midgut of the bumblebee B. terrestris and known to be competitive for resource consumption reduce the development of the natural parasite of this insect (the protozoan Crithidia bombi ) by 25% to 85% [102][118].
4. Impact of Yeasts and Their Volatile Compounds on Insect Behavior
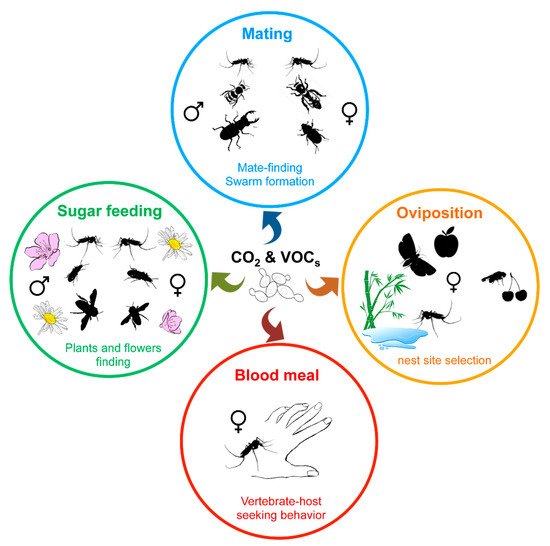
Besides visual signals, insects largely use the olfactory perception of chemical signals, such as emissions of CO 2 and pheromones or volatile organic compounds (VOCs), to move toward or find a partner, a food source (nectar, blood, etc.) or a nest site ( Figure 3 ) [87][103][104][105][106][104,136,137,138,139]. While plants, vertebrate hosts, or insects themselves directly produce such chemical compounds, environmental microorganisms or insect microbiota also contribute to the release of such kairomones. Indeed, CO 2 as along with a wide variety of volatile secondary metabolites are emitted by yeasts as by-products of fermentation, and play a role in insect attraction [87][107][104,140].
The ability to synthesize and release volatile compounds is also an old phenotypic trait that has been preserved in yeasts [108][141]. Several studies have shown that the simultaneous presence of VOCs and CO 2 both produced by yeasts during the fermentation of various carbon sources is more effective to attract insects than inert yeasts, industrial CO 2, or octenol (aromatic compound of plant or fungal origin widely used in commercial traps to capture biting insects) used alone [109][110][111][112][142,143,144,145]. For example, it was recently shown that the yeast Cyberlindnera jadinii adult attracted more efficiently green lacewing adults ( Chrysoperla comanche ) when it was alive, thus demonstrating the importance of the volatile compounds emitted by yeasts to attract these insects [113][146].
Blood-sucking insects such as mosquitoes which feed on both nectar (males, females) and blood (gravid females require blood meals to complete oogenesis), locate their food sources through volatile compounds (CO 2 and VOCs) partly emitted by yeasts found in plant nectar and on the skin of vertebrate hosts [105][138]. However, unlike nectar-living yeasts, the attractiveness of the yeasts found on human or vertebrate skins has never been tested. Depending on the nature of the VOCs generated and their concentration, attraction and repulsion behaviors have been observed towards mosquitoes [114][148]. Even if the fermentation by yeasts of complex carbohydrates such as honey generates a greater production of VOCs, including attractant compounds such as hexanoic acid or phenylethyl alcohol, sucrose attracts a greater number of mosquitoes. In this case, the absence of certain VOCs with repulsive properties could promote the attraction of mosquitoes [114][148]. In addition to their impact on the behavior of adult mosquitoes, yeasts also impact the feeding behavior of larvae. Yeasts that promote the development of larvae, through the supply of nutrients or the accumulation of reserves following the detection of a gut hypoxic signal [92][97][108,116], attract and strongly impact the behavior of larvae [115][116][149,150]. Indeed, the presence of S. cerevisiae in the larval food of Anopheles gambiae reduces the average velocity, rotations, and number of movements of larvae, while increasing their resting time [116][150].
A recent study has demonstrated that yeasts isolated from flowers, leaves, or fruits emitted specific VOC profiles that influence the feeding behavior of larvae of the moth Spodoptera littoralis . These larvae feed exclusively on leaves and are strongly attracted by yeasts retrieved from the plant phyllosphere ( Metschnikowia lopburiensis and Papiliotrema nemorosus ), while most of the yeasts isolated from fruits ( M. andauensis and M. pulcherrima ) are repellent. The attractive VOCs emitted specifically by the yeasts of the plant phyllosphere are geranyl acetone, cyclohexanone, 2-thyl-1-benzofuran, and 1,3,5-undecatriene [117][151].
