Carbon dots (CDs) have been identified as a promising class of photosensitiser nanomaterials for the specific detection and inactivation of different bacterial species. CDs possess exceptional and tuneable chemical and photoelectric properties that make them excellent candidates for antibacterial theranostic applications, such as great chemical stability, high water solubility, low toxicity and excellent biocompatibility.
1. Introduction
Antimicrobial resistance (AMR) has become a major threat that affects public health. One of the main causes for this problem is the extensive and disproportionate use of antimicrobial agents, which has led to the selection of drug-resistant pathogens that have developed new resistance mechanisms. The fast evolution of new AMR machineries, combined to the slow development and low approval rate of new drugs, has resulted in a major global health crisis [1][2][1,2]. Multidrug resistant bacteria isolated in hospitals represent an increasing risk factor especially for surgery and intensive care unit patients [3], making it harder, if not impossible, to treat infections with the consequent increase of medical complications and sanitation costs [4][5][4,5].
The development of reliable, cheap, and fast strategies for determining the presence or absence of bacteria or identification of specific species/strains in patient samples could reduce inappropriate prescribing of antibiotics in primary and secondary care [6]. Similarly, the ability to target antimicrobials to specific pathogens could reduce the inappropriate use of broad-spectrum antibiotics, which, as mentioned earlier, drives the emergence of both antibiotic resistance and healthcare-associated infections [7]. Lastly, the targeted delivery of antibiotics directly to the surface of specific cells may enhance their antibacterial activity through increasing local concentration or stimulating intracellular uptake [8]. The issue of antibiotic targeting is of particular relevance when considering Gram-negative bacteria, as the outer membrane of these species presents a formidable barrier to ingress for many antibiotic classes, which reduces treatment options for these organisms and complicates the development of new antibiotics [9]. This is exemplified by the fact that few new agents effective against Gram-negative bacteria are currently in clinical development. For example, oxazolidinones represent the first new chemical class of antibiotic to reach the clinic in over 30 years. These molecules are inhibitors of bacterial protein biosynthesis and represent an important class of drugs that are effective against a range of Gram-positive bacteria including multiresistant pathogens such as methicillin-resistant Staphylococcus aureus (MRSA). However, linezolid, the first such agent to reach the clinic, is becoming compromised by the emergence of resistance [10]. Thus, the prevention of infections through the early detection of pathogens and the development of new antibacterial agents able to circumvent bacterial multidrug resistance (MDR) represent a crucial objective of current biomedical research.
One of the most promising strategies in the fight against MDR pathogens entails the photodynamic inactivation of bacteria promoted by photosensitisers. This strategy relies on the light-promoted generation of highly reactive oxygen species (ROS) able to inactivate bacterial cells in different ways, including membrane destruction and/or irreversible protein and DNA damage [11][12][13][11,12,13]. The most important feature of photodynamic therapies consists of the generation of a closely localized physicochemical environment that is harsh to bacterial cells in ways such as ROS production or temperature increases (known as photothermal (PTT) effects), against which it is difficult to generate a resistance [14][15][14,15]. Among the different kind of photosensitisers, carbon dots (CDs) have emerged as a promising class of nanomaterials for the specific detection and inactivation of different bacterial species [16][17][16,17]. CDs are a class of quasispherical carbon-based fluorescent nanomaterials with a typical size of 10 nm or below. These materials possess great chemical stability, high water solubility, and outstanding photoelectric properties. In addition, they exhibit low toxicity and excellent biocompatibility [18]. These features, together with their ease of preparation and reduce material costs, makes CDs ideal candidates for antibacterial theranostic applications. Indeed, since their serendipitous discovery in 2004 by Xu et al. [19], CDs have found further applications across many scientific disciplines including semiconductors [20][21][20,21], biomedicine [22][23][22,23] catalysis [24], sensing and functional materials [25][26][25,26], and in the agricultural field [27][28][27,28].
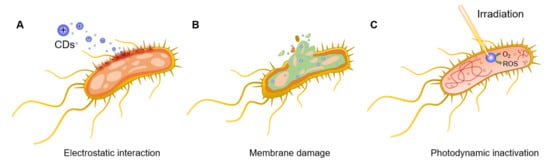
2. General Antibacterial Mechanisms of Action of CDs
The CD’s functional and biological properties are directly linked to the nanomaterial core structure and, in particular, to the functional groups exposed on the CD surface. Moreover, CD structure is strictly dependent on the choice of precursors and synthetic methods adopted during the manufacturing process. Thus, small differences in terms of type of precursors, solvents, and synthetic approaches lead to the formation of structurally different nanoparticles. This makes it difficult to predict the potential antibacterial efficacy and specificity for any novel CD without undertaking exhaustive structural characterization studies.
For instance, Bing et al.
[29][45] demonstrated that the surface charges of CDs play a pivotal role in the initial interaction with bacterial species and, thus, in their fluorescent labelling. Positively charged CDs interact via electrostatic interactions with the negatively charged
Escherichia coli (
E. coli) cell wall, promoting nanoparticle internalization and bacterial apoptosis (
Figure 2A). This initial interaction step is achieved thanks to the presence of ammonium on the surface of CDs and carboxyl and phosphate salts on the bacterial cell wall
[30][46]. The bacteriostatic or bactericidal effect of CDs can be exerted through several major routes, including physical and mechanical damage to the bacterial membrane, destruction of bacterial cell wall with consequent leakage of cytoplasmic material (
Figure 2B)
[31][47], inactivation via PTT effects due to localized temperature increase
[15], direct or light promoted generation of ROS
[32][33][48,49], and DNA and protein damage and fragmentation (
Figure 2C)
[34][50]. Bacterial inactivation promoted by membrane damage is also a commonly observed consequence of CD intercalation in the bacterial membrane
[29][35][45,51]. Additionally, N-doped CDs can be used as photosensitizers for the generation of ROS under UV or visible light irradiation, which leads to bacterial oxidative stress via the formation of H
2O
2, hydroxyl radicals (·OH), a superoxide anion (·O
2−), and singlet oxygen (
1O
2) upon reaction with water and dissolved O
2. PTT effects using near infrared (NIR) laser absorption are often exploited as a mechanism to activate the nanoparticles and induce a localized increase in temperature with consequential bacterial death. To this purpose, CDs doped with transition metals within their core structure (or via the formation of CD/metal nanoparticle composites) have been shown to be effective strategies to increase NIR PTT effects
[36][52]. Finally, positively charged CDs are also able to bind bacterial DNA and RNA molecules, leading to the fragmentation of the genetic material and subsequent cellular inactivation, as demonstrated in the inactivation of
E. coli and
Staphylococcus aureus (
S. aureus) models
[37][53].
Figure 2. General bactericidal mechanisms of action of CDs. (A) Schematic representation of the initial electrostatic interaction between CDs and the bacterial cell wall. (B) CDs internalization, intercalation in the bacterial membrane, and irreversible disruption with a leak of cytoplasmatic material. (C) CD-promoted bacterial photodynamic inactivation with ROS production and DNA damage.
3. CDs as Bacteria Targeting and Antibacterial Agents
3.1. Applications of CDs as Labelling and Bactericidal Agents (Theranostics)
In many recent examples CDs found successful applications in both the labelling and eradication of different bacterial species including both Gram-positive and Gram-negative models. Among the different characterization techniques, fluorescence microscopy and ζ-potential analysis constitute the preferred methods to determine the effective interaction, labelling, and internalization of CDs with bacteria. In spite of the difference in the cell wall composition of Gram-positive and Gram-negative bacteria, they all present an overall negative charge at the bacterial surface that promotes the initial electrostatic interaction with positively charged CDs. The effectiveness of CDs as antibacterial agents is typically determined via a series of techniques such as a disk diffusion assay, determination of the bacterial optical density (OD), and colony-forming unit (CFU) counting, among others. Finally, scanning electron microscopy (SEM), detection of ROS production, and proteomic analysis, represent the most frequently used techniques for the determination of the mechanism of action of CDs. It is important to remark that small changes in the CD precursors and synthetic approaches will lead to different king of nanoparticles. This may result in substantial changes in the CDs’ bacterial labelling specificity and bactericidal activity, making impossible to predict if a novel type of nanoparticle will selectively interact with Gram-positive or Gram-negative bacterial species. Therefore, a comprehensive use of the aforementioned techniques is required to unveil the CDs’ antibacterial mechanism of action and specificity toward different bacterial species.
3.2. Applications of CDs for Biofilm Eradication and Inhibition
Bacterial biofilm is a microenvironment generated by most bacterial species to protect themselves from external agents (such as adverse environmental and biochemical conditions) that leads to an increased bacterial survival rate
[38][94]. Biofilms are constituted from clusters of microorganisms held together in a selfproduced matrix made of proteins, polysaccharides, and environmental DNA (eDNA). Protected within the biofilm environment, pathogenic bacteria become a growing threat for human health, especially in the case of chronic infections. The biofilm matrix promotes bacterial infections by improving colonies’ adhesion to surfaces by creating a favourable microenvironment for bacteria to grow and by physically shielding bacterial cells from the immune system and the action of antibiotics
[39][95]. Owing to their small size, CD nanoparticles have been shown to be able to penetrate biofilm matrices and eradicate or prevent its formation in both Gram-positive and Gram-negative bacteria both achieving broad antibiofilm action
[40][78], as well as specific targeting towards Gram-positive
[41][42][55,96] or Gram-negative
[43][97] bacteria. Otis et al.
[44][98] recently prepared aminoguanidine/citric acid-based CDs able to selectively label Gram-negative
P. aeruginosa (but not
S. aureus) and to inhibit
P. aeruginosa biofilm formation. The authors found a correlation between the N-content doping and the bactericidal effect against
P. aeruginosa. Similarly, CDs prepared from
Artemisia argyi leaves could selectively inhibit
E. coli over
S. aureus biofilm formation, promoting cell death via membrane intercalation and damage
[45][99].
4. Conclusions
The emergence of antimicrobial resistance represents a significant health and economic challenge worldwide. The slow pace of antibacterial discovery requires the development of novel antimicrobial drugs, but it also requires the development of improved strategies for the repurposing of existing agents and effective diagnostic tools that can inform antibiotic prescription. In recent years, the development of novel probes able to target bacteria for detection and killing as effective theranostic strategies has grown into a focus of great importance. Carbon dots (CDs) have emerged as promising bioimaging probes due to their many advantages over molecular fluorophores and other fluorescent nanoparticles, since the preparation of these water-soluble carbon-based nanomaterials is often practical and low-cost.
Since the CD’s functional and biological properties are linked to the nanomaterial molecular structure which is dependent on the choice of starting materials and synthetic strategy, it has become evident that in order to develop tailored carbon-based nanomaterials for bespoke applications, more efforts are still needed to define the key parameters required to develop robust and reproducible synthetic strategies that lead to homogeneous materials with defined molecular features. Not surprisingly, we now appreciate that not all carbon dots are the same and that their structural features (e.g., type of core and surface functionalities) have a significant effect on how these materials interact with other cells and organisms, and this can be exploited to design probes that can target specific bacterial classes (such as Gram-negative or Gram-positive bacteria).