The Maillard reaction is a simple but ubiquitous reaction that occurs both in vivo and ex vivo during the cooking or processing of foods under high-temperature conditions, such as baking, frying, or grilling. Glycation of proteins is a post-translational modification that forms temporary adducts, which, on further crosslinking and rearrangement, form permanent residues known as advanced glycation end products (AGEs). Cooking at high temperature results in various food products having high levels of AGEs. This review underlines the basis of AGE formation and their corresponding deleterious effects on the body. Glycated Maillard products have a direct association with the pathophysiology of some metabolic diseases, such as diabetes mellitus type 2 (DM2), acute renal failure (ARF), Alzheimer’s disease, dental health, allergies, and polycystic ovary syndrome (PCOS). The most glycated and structurally abundant protein is collagen, which acts as a marker for diabetes and aging, where decreased levels indicate reduced skin elasticity. In diabetes, high levels of AGEs are associated with carotid thickening, ischemic heart disease, uremic cardiomyopathy, and kidney failure. AGEs also mimic hormones or regulate/modify their receptor mechanisms at the DNA level. In women, a high AGE diet directly correlates with high levels of androgens, anti-Müllerian hormone, insulin, and androstenedione, promoting ovarian dysfunction and/or infertility. Vitamin D3 is well-associated with the pathogenesis of PCOS and modulates steroidogenesis. It also exhibits a protective mechanism against the harmful effects of AGEs.
- advanced glycation end products
- Maillard reaction
- diabetes
- Alzheimer’s disease
- polycystic ovarian syndrome
- infant formula
1. Introduction: A Brief Glance at Advanced Glycation End Products (AGEs)
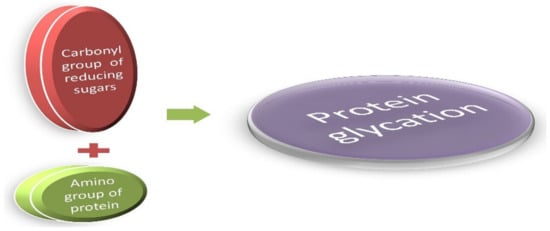
2. Advanced Glycation End Products (AGEs) and Modern Diet
-
CML: Nε-carboxymethyl-lysine;
-
Mass spectrometry;
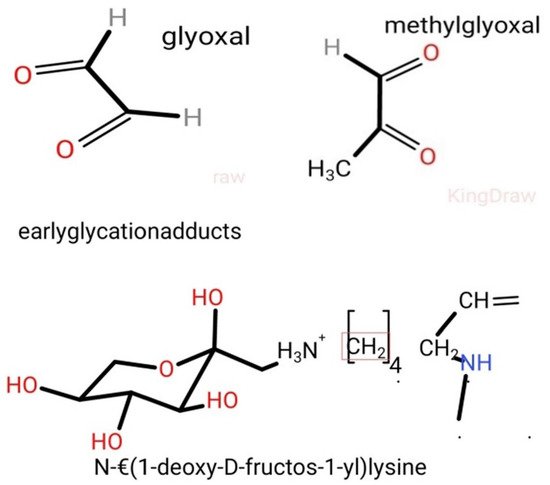
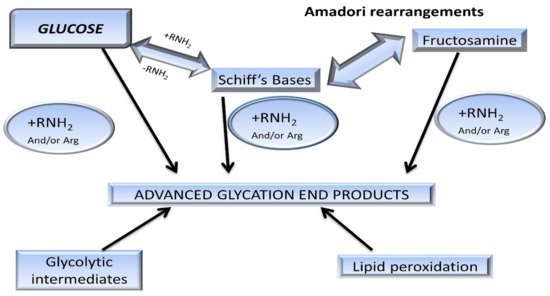
3. AGEs: Formation and Absorption
- CEL: Nε-1-carboxyethyl-lysine;
-
Pyrraline;
-
Glyoxal;
-
Methylglyoxal;
-
Acrylamide;
-
Derivatives of bis(lysyl)imidazolium:
-
DOLD: Deoxyglucosone-derived lysine dimer[1,3-di(Nε-lysino)-4(2,3,4-trihydroxybutyl)-imidazolium salt]
-
GOLD: glyoxal-derived lysine dimer[1,3-di(Nε-lysino) imidazolium salt].
-
References
- Steinhart, H. The Maillard Reaction. Chemistry, Biochemistry and Implications. By Harry Nursten. Angew. Int. Ed. 2005, 44, 7503–7504.
- Delgado-Andrade, C.; Fogliano, V. Dietary Advanced Glycosylation End-Products (dAGEs) and Melanoidins Formed through the Maillard Reaction: Physiological Consequences of their Intake. Annu. Rev. Food Sci. Technol. 2018, 9, 271–291.
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281.
- Munch, G.; Schicktanz, D.; Behme, A.; Gerlach, M.; Riederer, P.; Palm, D.; Schinzel, R. Amino acid specificity of glycation and protein-AGE crosslinking reactivities determined with a dipeptide SPOT library. Nat. Biotechnol. 1999, 17, 1006–1010.
- Nowotny, K.; Schroter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 47, 55–66.
- Vasan, S.; Foiles, P.; Founds, H. Therapeutic potential of breakers of advanced glycation end product-protein crosslinks. Arch. Biochem. Biophys. 2003, 419, 89–96.
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429.
- Nass, N.; Bartling, B.; Navarrete Santos, A.; Scheubel, R.J.; Borgermann, J.; Silber, R.E.; Simm, A. Advanced glycation end products, diabetes and ageing. Z. Gerontol. Geriatr. 2007, 40, 349–356.
- Makita, Z.; Vlassara, H.; Cerami, A.; Bucala, R. Immunochemical detection of advanced glycosylation end products in vivo. J. Biol. Chem. 1992, 267, 5133–5138.
- Kasper, M.; Funk, R.H. Age-related changes in cells and tissues due to advanced glycation end products (AGEs). Arch. Gerontol. Geriatr. 2001, 32, 233–243.
- Ko, S.Y.; Ko, H.A.; Chu, K.H.; Shieh, T.M.; Chi, T.C.; Chen, H.I.; Chang, W.C.; Chang, S.S. The Possible Mechanism of Advanced Glycation End Products (AGEs) for Alzheimer’s Disease. PLoS ONE 2015, 10, e0143345.
- Pinkas, A.; Aschner, M. Advanced Glycation End-Products and Their Receptors: Related Pathologies, Recent Therapeutic Strategies, and a Potential Model for Future Neurodegeneration Studies. Chem. Res. Toxicol. 2016, 29, 707–714.
- Juranek, J.; Ray, R.; Banach, M.; Rai, V. Receptor for advanced glycation end-products in neurodegenerative diseases. Rev. Neurosci. 2015, 26, 691–698.
- Ahmed, N.; Argirov, O.K.; Minhas, H.S.; Cordeiro, C.A.; Thornalley, P.J. Assay of advanced glycation endproducts (AGEs): Surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine-and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem. J. 2002, 364, 1–14.
- Ravichandran, G.; Lakshmanan, D.K.; Raju, K.; Elangovan, A.; Nambirajan, G.; Devanesan, A.A.; Thilagar, S. Food advanced glycation end products as potential endocrine disruptors: An emerging threat to contemporary and future generation. Environ. Int. 2019, 123, 486–500.
- Rutkowska, A.Z.; Diamanti-Kandarakis, E. Polycystic ovary syndrome and environmental toxins. Fertil. Steril. 2016, 106, 948–958.
- Kutlu, T. Dietary glycotoxins and infant formulas. Turk Pediatr. 2016, 51, 179–185.
- Nowotny, K.; Jung, T.; Hohn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222.
- Baye, E.; de Courten, M.P.; Walker, K.; Ranasinha, S.; Earnest, A.; Forbes, J.M.; de Courten, B. Effect of dietary advanced glycation end products on inflammation and cardiovascular risks in healthy overweight adults: A randomised crossover trial. Sci. Rep. 2017, 7, 4123.
- Vlassara, H.; Fuh, H.; Makita, Z.; Krungkrai, S.; Cerami, A.; Bucala, R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: A model for diabetic and aging complications. Proc. Natl. Acad. Sci. USA 1992, 89, 12043–12047.
- Gomez-Ojeda, A.; Jaramillo-Ortiz, S.; Wrobel, K.; Wrobel, K.; Barbosa-Sabanero, G.; Luevano-Contreras, C.; de la Maza, M.P.; Uribarri, J.; Del Castillo, M.D.; Garay-Sevilla, M.E. Comparative evaluation of three different ELISA assays and HPLC-ESI-ITMS/MS for the analysis of N(epsilon)-carboxymethyl lysine in food samples. Food Chem. 2018, 243, 11–18.
- Wellner, A.; Huettl, C.; Henle, T. Formation of Maillard reaction products during heat treatment of carrots. J. Agric. Food Chem. 2011, 59, 7992–7998.
- Zhou, Y.; Lin, Q.; Jin, C.; Cheng, L.; Zheng, X.; Dai, M.; Zhang, Y. Simultaneous analysis of Nε-(carboxymethyl)Lysine and Nε-(carboxyethyl)lysine in foods by ultra-performance liquid chromatography-mass spectrometry with derivatization by 9-fluorenylmethyl chloroformate. J. Food Sci. 2015, 80, C207–C217.
- Zhang, Y.; Cocklin, R.R.; Bidasee, K.R.; Wang, M. Rapid determination of advanced glycation end products of proteins using MALDI-TOF-MS and PERL script peptide searching algorithm. J. Biomol. Tech. 2003, 14, 224–230.
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21.
- Paul, R.G.; Bailey, A.J. Glycation of collagen: The basis of its central role in the late complications of ageing and diabetes. Int. J. Biochem. Cell Biol. 1996, 28, 1297–1310.
- Uribarri, J. Dietary AGE and Their Role in Health and Disease; CRCPress: BocaRaton, FL, USA, 2018.
- Koschinsky, T.; He, C.-J.; Mitsuhashi, T.; Bucala, R.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479.
- Snelson, M.; Coughlan M., T. Dietary Advanced Glycation End Products: Digestion, Metabolism and Modulation of Gut Microbial Ecology. Nutrients 2019, 11, 215.
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of age formation. Food Funct. 2016, 7, 46–57.
- Grunwald, S.; Krause, R.; Bruch, M.; Henle, T.; Brandsch, M. Transepithelial flux of early and advanced glycation compounds across caco-2 cell monolayers and their interaction with intestinal amino acid and peptide transport systems. Br. J. Nutr. 2006, 95, 1221–1228.
- Hellwig, M.; Matthes, R.; Peto, A.; Lobner, J.; Henle, T. N-epsilon-fructosyllysine and n-epsilon-carboxymethyllysine, but not lysinoalanine, are available for absorption after simulated gastrointestinal digestion. Amino Acids. 2014, 46, 289–299.
- Teodorowicz, M.; Neerven, J.V.; Savelkoul, H. Food Processing: The Influence of the Maillard Reaction on Immunogenicity and Allergenicity of Food Proteins. Nutrients 2017, 9, 835.
- Bergmann, R.; Helling, R.; Heichert, C.; Scheunemann, M.; Mäding, P.; Wittrisch, H.; Johannsen, B.; Henle, T. Radio fluorination and positron emission tomography (PET) as a new approach to study the in vivo distribution and elimination of the advanced glycation endproducts N-epsilon-carboxymethyllysine (CML) and N-epsilon-carboxyethyllysine (CEL)and N epsilon-carboxyethyllysine (CEL). Nahrung 2001, 45, 182–188.
- He, C.; Sabol, J.; Mitsuhashi, T.; Vlassara, H. Dietary glycotoxins: Inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes 1999, 48, 1308–1315.
