1. Overview
Lung cancer is one of the most common malignancies with the highest mortality rate and the second-highest incidence rate after breast cancer, posing a serious threat to human health. The accidental discovery of the antitumor properties of cisplatin in the early 1960s aroused a growing interest in metal-based compounds for cancer treatment. However, the clinical application of cisplatin is limited by serious side effects and drug resistance. Therefore, other transition metal complexes have been developed for the treatment of different malignant cancers. Among them, Ru(II/III)-based complexes have emerged as promising anticancer drug candidates due to their potential anticancer properties and selective cytotoxic activity. In this review, we summarized the latest developments of Ru(II/III) complexes against lung cancer, focusing mainly on the mechanisms of their biological activities, including induction of apoptosis, necroptosis, autophagy, cell cycle arrest, inhibition of cell proliferation, and invasion and metastasis of lung cancer cells.
2. Primary Bronchogenic Carcinoma
Primary bronchogenic carcinoma, also known as lung cancer, is a malignant tumor that originates from the bronchial mucosa or gland. With a high incidence and mortality rate, lung cancer poses a serious threat to human health, while its cases and deaths rise every year
[1]. Although its incidence follows breast cancer, lung cancer remains the leading cause of cancer death, with approximately 1.8 million deaths (18%) worldwide
[2]. Moreover, the global cancer burden is expected to increase by 47% in 2040 compared to 2020, reaching a total of 28.4 million cases
[2]. However, current advances in novel chemotherapeutic agents, targeted therapies, standardized diagnosis, and staging and multidisciplinary treatment of lung cancer have improved patient survival rates
[3]. Nevertheless, the prognosis of lung cancer patients is still poor due to insufficient early diagnosis.
Lung cancer can be classified into central and peripheral lung cancer depending on the anatomical part affected, as well as into two main pathological entities: non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). NSCLC can be further subdivided into three histological subtypes: lung squamous cell carcinoma, lung adenocarcinoma, and large cell carcinoma. NSCLC, which accounts for about approximately 85% of lung cancer cases, has shown an increased mortality rate in recent years. Radical surgery is the most common treatment applied to early-stage NSCLC patients, while chemotherapy is mainly used for NSCLC patients in advanced or recurrent stages
[3]. Moreover, NSCLC patients with unresectable tumors in the advanced stage are still treated with Pt-based doublet chemotherapies such as cisplatin–etoposide and carboplatin–paclitaxel
[4]. Currently, chemotherapy combined with Pt-based antineoplastic agents, such as cisplatin, oxaliplatin, and carboplatin, has been efficiently used to treat various cancers, including NSCLC. However, lung cancer patients show different sensitivity to Pt-based chemotherapy and 20–40% of them tend to relapse within six months after treatment
[5]. It is also known that the anticancer activity of cisplatin targeting nuclear DNA is based on the formation of cisplatin–DNA adducts, which stop DNA replication and transcription, while triggering cancer cell apoptosis
[6][7][6,7]. However, tumor resistance to cisplatin reduces the accumulation of drugs in cancer cells, rapid DNA repair, and upregulation of transcription factors
[8], thus significantly limiting its clinical application. Moreover, Pt drugs can lead to serious side effects, such as nephrotoxicity, ototoxicity, nausea, vomiting, hair loss, etc., further limiting their effective use
[8][9][8,9]. Therefore, researchers have focused on the development of alternative anticancer drugs to overcome the drawbacks of Pt-based agents in NSCLC patients.
Considering the effectiveness of cisplatin and its derivatives, other transition-metal complexes, such as Ru-, Ir-, Rh-, Pd-, Au-, and Os-based complexes, have emerged as a new generation of promising anticancer agents due to their potential anticancer properties and selective cytotoxic activity
[10][11][12][13][10,11,12,13]. Among them, Ru complexes have received particular attention owing to their good biodistribution and multimodal actions. Moreover, Ru compounds can effectively bind to the serum transferrin receptor, which is highly expressed in tumor cells, thus increasing the number of Ru–transferrin complexes that could preferably be delivered at the tumor site
[14][15][14,15]. Ru can be found in two stable oxidation states (II and III)
[16], which can coordinate with ancillary ligands of different geometries to prepare diverse Ru(II/III) complexes with different steric and electronic properties
[17]. For example, arene has been widely used as a ligand, as it can stabilize the oxidation state of metal complexes. Hence, a series of hydrophilic and hydrophobic arene Ru(II/III) complexes have been designed and synthesized with great potential for the development of metal-based chemotherapeutic drugs
[18][19][18,19]. There are few studies focused on Ru(IV) complexes that search for efficient anticancer candidates. Of note are the recent research reported by Lu, Y et al. who have proposed a novel dual-targeting Ru(IV) candidate with antitumor and antimetastatic properties in vitro and in vivo studies via the PARP/ATM pathway
[20]. There are different signaling pathways that participate in the anticancer activity of various Ru complexes, including the mitochondria-mediated pathway, the DNA damage-mediated pathway, and the death receptor-mediated pathway
[21][22][21,22].
Ru complexes can also trigger phototoxicity in cells, which induces a series of photochemical and photobiologic processes, leading to irreversible photodamage in tumor tissues
[16]. Thus, Ru compounds, such as Ru(II) polypyridyl complexes, which could interact with bovine serum albumin (BSA) and with DNA via minor grooves
[23][24][25][26][23,24,25,26], are considered attractive photo-mediated activation prodrugs for photodynamic therapy (PDT) and photoactivated chemotherapy (PACT)
[27]. Given also that photofrin, a hematoporphyrin derivative, is the only PDT drug approved by the Food and Drug Administration for cancer therapy, including early and advanced lung cancer
[28][29][28,29], extensive studies have been performed to develop novel Ru(II/III) complexes for efficient cancer treatment
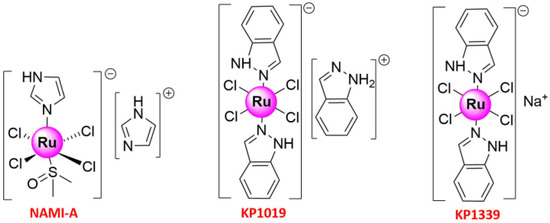
[16][18][30][16,18,30]. Metal-based anticancer candidate imidazolium [
trans-RuCl
4(1
H-imidazole) (DMSO-S) (
NAMI-A; DMSO = dimethyl sulfoxide) (
Figure 1) was the first Ru compound to be studied on human beings, which has reached the phase II stage
[31][32][33][34][31,32,33,34]. The study was launched in 2008 when
NAMI-A administered in combination with gemcitabine was given to patients with advanced NSCLC
[34]. Previously, fundamental works have shown that its lower molar cytotoxicity over cisplatin results from its reduced reactivity against DNA in intact cells, and more studies in animal models have exhibited the excellent and selective activity against lung metastases of some solid metastasizing tumors at a concentration with relatively mild toxicity
[32][33][35][32,33,35]. The other two important promising Ru(III) complexes that have entered clinical trials were indazolium [
trans-RuCl
4(1
H-indazole)
2] (
KP1019)
[36][37][38][39][36,37,38,39] and sodium [
trans-RuCl
4(1H-indazole)
2] (
KP1339)
[40] (
Figure 1). The pharmacokinetics of
KP1019, which was characterized by a small volume of distribution, low clearance, and long half-life, has been researched in a phase I dose-escalation study, in which five out of six patients treated with
KP1019 experienced disease stabilization with no severe side effects
[36][39][36,39]. With these pioneering works, Ru complexes are attracting increasing attention from chemical researchers. The recent years have witnessed the development of Ruthenium complexes as second-generation metal-based anticancer agents, possessing high potency of targeting cancer cells due to their low toxicity, the ability to induce apoptosis, selective anti-invasion, and anti-metastasis activity
[41][42][43][41,42,43]. Some of them have also shown anti-angiogenic properties and could therefore be used to inhibit angiogenesis, the basis of tumor growth and metastasis
[44]. Moreover, with the purpose of improving their in vivo stability, solubility, cellular uptake, and effectiveness, some research groups with meticulous design have developed special drug delivery systems (nanoparticles, liposomes, etc.), which could encapsulate Ru-based compounds appropriately
[30][45][46][47][30,45,46,47].
Figure 1. Structures of three important Ruthenium complexes entering clinical trials.
Based on these promising results, in this review, we summarize the recent findings on the anticancer mechanisms of Ru-based compounds targeting lung cancer, including apoptosis, autophagy, necroptosis, anti-metastasis, and cell cycle arrest.
3. Conclusions
Lung cancer remains a life-threatening malignancy due to poor prognosis and drug resistance, which is one of the most severe challenges that still need to be addressed to improve patients’ prognosis and survival rate. However, specific drugs preventing tumor metastasis and recurrence have not yet been efficiently developed. In order to design and synthesize effective anticancer agents, transition metal-based compounds have gradually evolved as promising drug candidates due to their cytotoxicity and ability to prevent drug resistance in tumor cells. Among them, Ru(II/III)-based compounds proved to be the most effective, as they show high cytotoxicity, which induces apoptosis, necroptosis, or autophagy and cell cycle arrest, thus inhibiting cell proliferation, invasion, and metastasis. Most studies suggested that Ruthenium complexes were low in toxicity, easily absorbed, and excreted quickly. More importantly, Ruthenium complexes were easily absorbed by tumor tissues.