Melanoma is the deadliest type of skin cancer, due to its invasiveness and limited treatment efficacy. The main therapy for primary melanoma and solitary organ metastases is wide excision. Adjuvant therapy, such as chemotherapy and targeted therapies are mainly used for disseminated disease. Radiotherapy (RT) is a powerful treatment option used in more than 50% of cancer patients, however, conventional RT alone is unable to eradicate melanoma. Its general radioresistance is attributed to overexpression of repair genes in combination with cascades of biochemical repair mechanisms. A novel sophisticated technique based on synchrotron-generated, spatially fractionated RT, called Microbeam Radiation Therapy (MRT), has been shown to overcome these treatment limitations by allowing increased dose delivery. With MRT, a collimator subdivides the homogeneous radiation field into an array of co-planar, high-dose microbeams that are tens of micrometres wide and spaced a few hundred micrometres apart. Different preclinical models demonstrated that MRT has the potential to completely ablate tumours, or significantly improve tumour control while dramatically reducing normal tissue toxicity.
1. Introduction to Melanoma
Melanoma is a highly malignant type of skin cancer that develops from melanocytes, the cells that produce the UV-absorbing pigment melanin. Although melanoma constitutes only a minority of skin cancers, it is the deadliest
[1]. During the last 50 years, the incidence of melanoma has dramatically increased and is now growing faster than any other cancer type. Worldwide, there are estimated to be around three new cases per 100,000 inhabitants per year; however, this picture varies by country and region
[2]. The highest incidences are found in developed countries of the new world, such as Australia and New Zealand (around 35 new cases per 100,000 inhabitants yearly), but also in the US (approximately 30 new cases per 100,000 inhabitants were expected for 2020)
[1]. Risk factors for melanoma include sun exposure, number of nevi, history of sunburns (especially during childhood), and light skin colour amongst other factors such as a genetic predisposition to photosensitivity
[3].
Several subtypes of melanomas can be distinguished as mucosal, uveal, anorectal, meningeal, and the most common, cutaneous melanoma. Cutaneous findings such as superficial spreading, nodular, and lentigo maligna account for the vast majority of melanoma cases. The difference in prognosis between these subtypes is mainly determined by the stage of growth at the time of diagnosis
[4]. Non-cutaneous melanomas are rarer and associated with a worse prognosis due to the delay in primary tumour discovery, high rate of relapse, and metastatic disease
[5].
Most melanomas are detected clinically and primarily diagnosed by physical examination, dermoscopy, histopathology, and imaging, with the detection of circulating biomarkers being less crucial. Depending on the age of the patient and tumour thickness, sentinel lymph node biopsy is a powerful staging tool
[6]. In addition, approximately 10 percent of all melanomas are initially diagnosed in the lymph node and are referred to as “unknown primary”. They are presumed to originate in the lymph node itself from pre-existing nodal nevi
[7]. For all melanoma, early diagnosis is critical for improving prognosis as most complications with melanoma stem from metastases and their impact on the affected organ. Paraneoplastic syndromes may also occur, but at a much lower rate
[8].
The severity of the disease is classified using four different stages according to the American Joint Committee on Cancer (AJCC)
[9]. The staging system takes into consideration the thickness and ulceration status of the primary tumour as well as the presence of metastasis in the nearby lymph nodes and in distal sites. Stage I represents the lowest grade of severity defined by a small and localized malignancy, while stage IV is the highest grade of severity in which distant metastases are present and the clinical course is highly variable
[10].
The main therapy for primary cutaneous melanoma is wide local excision. Depending on tumour thickness, a clinical margin of 0.5–2 cm is recommended for the excision in order to prevent local tumour recurrence
[11]. To treat local or satellite metastases, surgical excision of the primary tumour and of metastases (in lymph nodes or distant organs) should always be performed whenever possible with curative intent. However, complete lymph node dissection is no longer recommended for patients with a positive sentinel lymph node in microscopic nodal disease, as there is no evidence that this improves prognosis. Nevertheless, in macroscopic nodal disease, complete lymph node dissection remains the key strategy
[12][13][12,13]. Adjuvant therapy, such as chemotherapy and targeted therapies (e.g., inhibition of BRAF and c-KIT), are also employed for the treatment of melanoma. Melanoma has been shown to be an immunogenic tumour with polymorphic immune cell infiltration
[14]. As a result, immunotherapies (e.g., IFN-α, interleukins) are also actively being explored, including novel immune checkpoint blockade and adoptive T cell therapy amongst others. Radiation therapy (RT) is another major therapeutic strategy for the treatment of many malignancies, however, it is often ineffective in the eradication of melanoma which is historically considered to be radioresistant. Here, we discuss the obstacles preventing efficient treatment of melanoma with RT, and how novel RT modalities, such as synchrotron generated, spatially fractionated microbeam radiation therapy (MRT) can overcome these obstacles. In particular, we discuss the role of conventional RT-induced immunity and the benefits of MRT’s stronger anti-tumour immune response. We present a comparative gene expression analysis from previously published studies of preclinical tumour models treated with MRT. The results of this analysis reveal a specific gene signature for an ‘MRT-induced immune effect’. Finally, we share our own experience about the successful treatment of mouse B16-F10 melanoma with MRT.
2. Melanoma and Radiation Therapy
2.1. Radioresistance of Melanoma and Conventional RT as a Treatment Strategy
The concept of melanoma radioresistance is based on early studies involving radiation cell-survival curves for rodent and human melanoma cell lines, which suggested an enhanced capacity to repair radiation-induced DNA damage
[15][16][15,16]. Genetic analysis of 60 patients’ metastatic and non-metastatic melanoma samples revealed differential overexpression of genes involved in DNA double-strand break (DSB)/interstrand crosslink (ICL) repair, DNA replication, telomere maintenance, checkpoint activation, and also in nucleotide excision repair (NER) and base excision repair (BER)
[17]. The overexpression of repair genes explains the resistance of melanoma to therapy. Another explanation was provided by Wu et al.
[18], who analysed the response of the metabolome following ionizing radiation (IR) in mouse B16 melanoma cells. The levels of glutamate, alanine, glycine, and choline increased in irradiated cells. With a series of biochemical reactions upon the catalysis of these enzymes, more glycine can be synthesized which indirectly generates tetrahydrofolic acid, an important coenzyme in the DNA synthesis process. This finding supports that radiation tolerance of B16 cells is related to their efficient DNA damage repair.
The radiosensitivity of different melanoma cell lines varies significantly
[19]. The radiation response in different tumours can be affected by multiple radiobiological parameters, such as hypoxic fraction, ability to deoxygenate, vascularization, cellular proliferation kinetics, number of tumour stem cells, and the inherent radiosensitivity of the tumour cells themselves
[19][20][19,20]. Melanoma radioresistance has been found to positively correlate not only with efficient DNA damage repair, but also with a high fraction of hypoxic cells
[21], long volume doubling time, slow growth, high cell loss, and low vascular density
[22]. Matchuk et al.
[23] attributed radioresistance of B16 melanoma to the efficient radiation response of a side population of tumour cells. In response to 3 Grays (Gy) of low-LET radiation, this population had less DNA DSBs compared to their differently located counterparts, were more quiescent, and had higher concentrations of nitric oxide (NO) which inhibits apoptosis.
Adjuvant RT has been shown to improve locoregional disease control in melanoma, but not overall survival, however, this still remains under investigation
[24][25][24,25]. Currently, RT is used if surgery cannot be performed, or at the site of lymphadenectomy in node-positive melanoma patients
[26]. Hypofractionation has been advocated as a promising treatment strategy. Early clinical investigations revealed that hypofractionation with higher doses (4–8 Gy per fraction) leads to better tumour control, compared to lower dose fractionation (2–3 Gy per fraction)
[27]. Nevertheless, in the study of Lugade et al.
[28], fractionated irradiation (3 Gy × 5) of mouse B16F10 tumours was only marginally effective compared to non-irradiated tumour-bearing controls, and a single dose of 15 Gy slowed tumour progression only at early stages after irradiation. Overall, advanced RT modalities, such as modern, hypofractionated radiation schedules, have not been shown to have an improved therapeutic impact. However, ablative RT modalities, such as stereotactic radiosurgery (SRS) and stereotactic body RT (SBRT) are commonly used in the management of in-transit or satellite metastases, preferably brain metastases
[29]. Radiation is also used in palliative settings such as for treating spinal cord compression or painful bone metastases
[29]. Complications from radiation in melanoma treatment, mainly fibrosis and oedema, seem to be clinically manageable. Most therapeutic guidelines suggest a case-by-case discussion of adjuvant treatment options after complete lymph node dissection
[29].
2.2. Spatially Fractionated RT Including Synchrotron-Generated MRT
Technological advancements in RT have contributed to the improved efficacy of cancer treatment. These advancements are increasing the therapeutic ratio by successfully eradicating tumours while improving normal tissue sparing. Historically used to treat large, bulky tumours, spatially fractionated radiotherapy (SFRT) is a type of external beam radiation treatment that allows for the delivery of higher dose fractions of radiation in order to achieve local tumour control with minimal toxicity
[30][31][30,31]. Clinically, SFRT was originally delivered using a homogenous beam of orthovoltage X-rays with a physical grid placed over the tumour resulting in non-homogeneous dose delivery
[30][31][30,31]. This GRID therapy allowed for the delivery of higher doses while reducing damage to surrounding normal tissues, thus achieving superior outcomes in the palliative treatment of advanced-stage malignancies (Reviewed by Yan et al.
[32]).
With advancements in technology, more sophisticated techniques were developed to deliver SFRT. GRID therapy could then be administered with megavoltage X-ray tubes where doses between 10 and 25 Gy were deliverable with minimal toxicity
[32][33][32,33]. LATTICE RT is the evolution of 2D GRID therapy to a 3D configuration where high doses are delivered as independent vertices of incident radiation
[34][35][34,35]. Distinct spatial configurations have also been developed to improve the therapeutic index of SFRT with millimeter and submillimeter fractionation. Minibeam RT delivers radiation as an array of parallel beams with widths of 0.5–0.7 cm spaced 1–3 mm apart using both proton
[36][37][36,37] and X-ray
[38][39][40][38,39,40] sources. Carbon nanotube technology is also being used to generate submillimetric beams with X-ray tubes
[41].
Another type of SFRT that adopts submillimetre fractionation is MRT. Experimental synchrotron X-ray-generated MRT is delivered with the same dose deposition pattern of the minibeams, but using sub-millimetric beam widths of 25–100 μm spaced 200–400 μm apart, which drastically increases the normal tissue tolerance to radiation doses in the range of hundreds of Gy. This greatly exceeds the thresholds of conventional RT and the previously discussed SFRT regimens (reviewed by Fernandez-Palomo et al.
[42] and Eling et al.
[43]). In order to produce microbeams of this geometry, a beam with minimal divergence delivered at ultra-high dose rates is required, and therefore synchrotron sources are necessary
[44]. X-ray microbeams of average 100 KeV beam energy (spectrum 50–600 keV) are delivered at dose rates of 10
4 Gy/s up to 16 kGy/s
[43][44][43,44]. Such dose rates trigger FLASH effects that confer additional biological benefits
[45]. The biological FLASH effect was found to be reproducible when the whole dose of radiation is delivered in less than 200 milliseconds
[46]. Synchrotron MRT is capable of generating the FLASH effect by delivering the highest peak doses compared to all other SFRT regimens within this time frame. Importantly, MRT promotes exceptional tumour control in conjunction with normal tissue sparing which has been well documented in a variety of models (reviewed in
[42]). This includes melanoma, where remarkable tumour control was achieved in a murine model of B16F10 melanoma
[47][48][47,48].
3. MRT as a Novel Strategy for Treatment of Melanoma
A recent research interest of our group is exploring the efficacy of MRT for treating melanoma. An ad hoc preclinical B16F10 radioresistant melanoma model for testing a microbeam array was established in our laboratory
[49][119]. In the very first study, it was clear that MRT elicits an extraordinary delay of tumour growth when compared to a BB irradiation or to un-irradiated controls
[47]. Aside from the underlined mechanisms of vascular disruption and induction of cellular senescence, we demonstrated that MRT elicits superior tumour control due to the induction of a potent tumour immune response. Especially, MRT induces a significant increase of monocyte-attracting cytokines (MCP-1, MIP-1a, MIP-1b, RANTES), and IL-12p40 in the melanoma microenvironment
[47]. MRT also promoted infiltration of macrophages, CD4+ and CD8+ T cells and NK cells, both in the periphery and within the irradiated tumour 5–12 days after treatment. This was observed in contrast to naïve or BB-irradiated tumours
[47].
In another study, Fernandez-Palomo and colleagues compared the efficacy of delivering three fractions of 133 Gy MRT, administered in three consecutive days versus only one MRT fraction of 400 Gy in a single day
[48]. Remarkably, after the temporally fractionated irradiation, 50% of melanomas underwent complete tumour remission. For an 18 months period, the mice showed no sign of tumour re-growth, and after they were sacrificed, immunohistochemical analysis of the tumoural vestige revealed a complete absence of melanoma cells and the presence of melanophages. Melanophages were described as very large melanin-laden cells, positive for macrophage markers
[48].
The importance of this result is not only that radioresistant B16F10 melanoma does not lose sensitivity to the radiation treatment after several fractions, but also that there is no local tumour recurrence and metastasis for a long period of time. This could be explained by the presence of the anti-tumour abscopal effect specifically triggered by fractionated MRT. Therefore, this work offers a unique model to further study MRT-induced abscopal effects and is a starting point for optimizing a treatment protocol that could increase the rate of tumour remission above 50% in this preclinical model.
In
Table 1, we summarize findings in the field of MRT-induced tumour immune responses in melanoma and other cancers that were reported in this review. In
Figure 2, we outline the advantages and disadvantages of conventional RT and MRT for the treatment of melanoma. In the case of MRT, the factors discussed above, such as the induction of the anti-tumour immune response, overcoming tumour radioresistance, and the ability to generate the anti-tumour abscopal effects, contribute to the treatment success in melanoma. We have yet to optimize the fractionation schedule on the basis of normal tissue tolerance and tumour response. In parallel, the success of MRT in melanoma treatment can be further boosted by exploiting the role of IR in the modulation of local and systemic immune processes. The treatment improvement will be based on enhancing the anti-tumour immune response in combination with MRT. This can be done directly by targeting immune checkpoints, or indirectly, by blocking the upstream of the innate (‘frontline’) immune response. The proof of concept of these treatment combinations will open a completely novel avenue for the treatment of radioresistant tumours beyond melanoma.
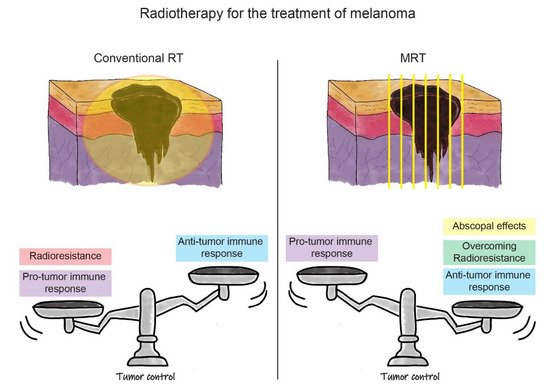
Figure 2. Schematic representation of the RT strategies for the treatment of melanoma. In the upper part of the figure, there is a schematic representation of melanoma irradiation with conventional RT (on the left) and MRT (on the right). In the lower part, the balance between positive and negative effects influencing treatment outcomes are represented (i.e., tumour control) for both RT modalities. On the left, we can see how the pro-tumour immune response, associated with radioresistance, outbalances the anti-tumour immune response induced by conventional RT. This leads to the current uncommon use of conventional RT for the treatment of melanoma. In the lower right panel, it is shown how the positive effects of MRT, such as induction of the anti-tumour immune response, overcoming melanoma radioresistance, and possible triggering of abscopal effects, shift the balance towards a better outcome for melanoma eradication. This promotes MRT as a new opportunity for melanoma treatment in future clinical settings.
Table 1. The most important findings in the field of MRT induced response in different tumour models.
| Tumor Model |
Assay Type |
MRT Effects on Tumor Immune Response |
Reference |
| Glioblastoma in rat |
Oligonucleotide microarray |
Upregulation of genes associated with inflammation, NK or CD8+ T cells |
Bouchet et al., 2013 [50] | Bouchet et al., 2013 [88] |
| Glioblastoma in rat |
Oligonucleotide microarray |
Upregulation of transcripts indicating the presence of DCs, monocytes, and macrophages |
Bouchet et al., 2014 [51] | Bouchet et al., 2014 [89] |
| Glioblastoma in rat |
IHC |
Increase of infiltrated macrophages |
Eling et al., 2021 [52] | Eling et al., 2021 [101] |
| Mammary EMT6.5 in mouse |
Whole genome analysis |
Upregulation of genes related to inflammation, IFN signalling,
antigen presentation |
Sprung et al., 2012 [53] | Sprung et al., 2012 [87] |
| Mammary EMT6.5 cell line |
Whole genome analysis |
Upregulation of pathways involved in inflammation and lymphocyte activation |
Yang et al., 2014 [54] | Yang et al., 2014 [90] |
| Mammary EMT6.5 in mouse |
Flow cytometry, IHC |
Decrease in tumour-associated macrophages and neutrophils;
increase of infiltrated T cells |
Yang et al., 2019 [55] | Yang et al., 2019 [96] |
| Melanoma B16F10 in mouse |
Cytokine BioPlex analysis, IHC |
Increase of monocyte-attracting cytokines; Increase of infiltrated macrophages, CD4+ and CD8+ T cells, NK cells |
Potez et al., 2019 [47] |
| Melanoma B16F10 in mouse |
IHC |
Presence of melanophages at the place of tumor cells and absence of metastasis up to 18 months post-treatment |
Fernandez-Palomo et al., 2020 [48] |