Diabetes mellitus (DM) is a prevailing global health metabolic disorder, with an alarming incidence rate and a huge burden on health care providers. DM is characterized by the elevation of blood glucose due either to a defect in insulin synthesis, secretion, binding to receptor, or an increase of insulin resistance. The internal and external factors such as obesity, urbanizations, and genetic mutations could increase the risk of developing DM. Flavonoids are phenolic compounds existing as secondary metabolites in fruits and vegetables as well as fungi. Their structure consists of 15 carbon skeletons and two aromatic rings (A and B) connected by three carbon chains. Flavonoids are furtherly classified into 6 subclasses: flavonols, flavones, flavanones, isoflavones, flavanols, and anthocyanidins. Naturally occurring flavonoids possess anti-diabetic effects. As in vitro and animal model’s studies demonstrate, they have the ability to prevent diabetes and its complications.The aim of this review is to summarize the current knowledge addressing the anti-diabetic effects of dietary flavonoids and their underlying molecular mechanisms on selected pathways: Glucose transporter, hepatic enzymes,tyrosine kinase inhibitor, AMPK, PPAR, and NF-B. Flavonoids improve the pathogenesis of diabetes and its complications through the regulation of glucose metabolism, hepatic enzymes activities, and lipid profile. Most studies illustrate a positive role of specific dietary flavonoids on diabetes, but the mechanisms of action and the side effects need more clarification. Overall, more research is needed to provide a better understanding of the mechanisms of diabetes treatment using flavonoids.
- diabetes mellitus
- flavonoids
- hyperglycemia
- anti-diabetic
- lipogenesis
1. Diabetes and Flavonoids
1.1. Diabetes Mellitus
1.2. Glucose Homeostasis
1.3. Insulin Resistance
1.4. Insulin Release Defect in Diabetes
1.5. Lipogenesis Regulation in Adipocytes
1.6. Diabetes Management
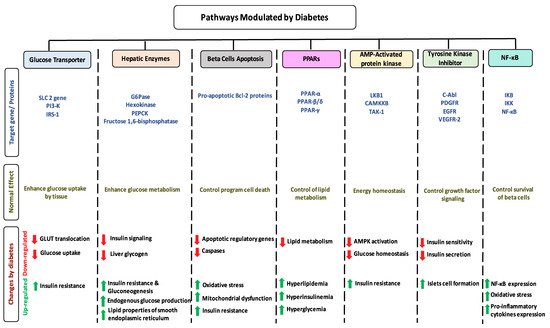
1.7. Impact of Diabetes on Selected Pathways
1.8. Dietary Flavonoids
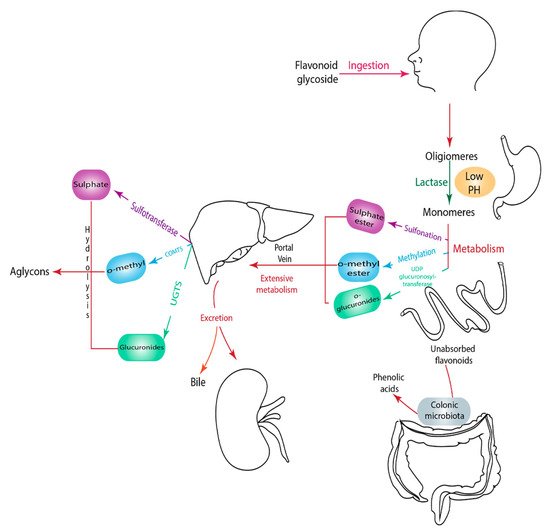
1.9. Metabolism of Flavonoids
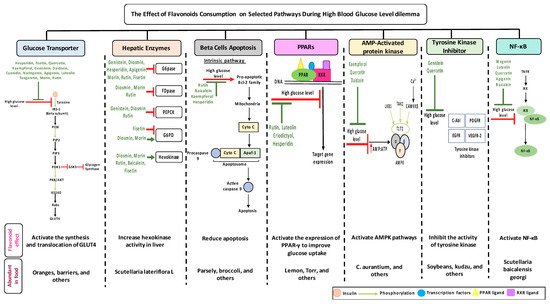
2. Anti-Diabetic Effects of Selected Flavonoids
2.1. Flavonol
2.1.1. Quercetin
| Flavonoid Subclass | Name of Flavonoid | Structure of Flavonoid | Dietary Source | Metabolites Produced from Flavonoids | Function of Flavonoids | Mechanism of Action | Model Used | References | |
|---|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||||||
| Flavonol | 1. Rutin | 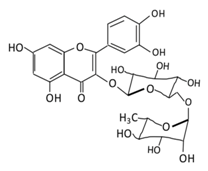 |
Oranges, grapes, limes, lemons, berries and peaches. | Metabolization depends on intestinal bacteria: (A) Bacillus 52 and Bacteroides 45 produce: Quercetin-3-O-glucoside and Leucocynaidin. (B) Bacteroides 42 and veillonella 32 produces: Leucocynaidin. (C) Bacteroides 22 hydrolysis produce: Quercetin-3-O-glucosie |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Inhibit α-glucosidase and α-amylase which reduce the absorption of glucose in small intestine Decrease G6Pase, PEPCK, glycogen phosphorylase, and fructose-1,6-bisphosphatase enzymes in liver and kidney Decrease the level of caspase 3 and increase the level of Bcl-2 which shows an anti-apoptotic activities Reduce the level of hemoglobin A1C (HbA1c) Activate the synthesis and translocation of GLUT4 that stimulate glucose transport to soleus muscle tissue Increase hexokinase activity in liver Improve the morphology of islets of Langerhans Reduce serum LDL, VLDL, triglyceride Inhibit lipid peroxidation Increase serum level of HDL Activate the expression of PPAR-γ which improve glucose uptake and insulin resistance |
Streptozotocin induced diabetic rats Type 2 diabetic rat Streptozotocin induced diabetic wistar rats |
Streptozotocin diabetic tissue |
[81,[8183]][82] |
| 2. Fisetin | 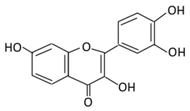 |
Onion, strawberries, and persimmon | (A) Glucuronide of fisetin (M1) (B) Glucuronide (M2) (C) Methoxylated metabolites of fisetin (M3) |
(A) Antihyperglycmeic effect | Inhibit gluconeogenesis by inhibiting pyruvate transport into mitochondria Decrease glycogen breakdown which prevent hyperglycemia Reduce blood glucose, Hb1Ac, IL-1β, and NF-κB p65 unit Reduce the activity of glucose glucose-6-phosphate dehydrogenase activity |
Streptozotocin induced diabetic rats |
[107,108][83][84] | ||
| 3. Kaempferol |  |
Cruciferous vegetables, tea, grapefruit, edible berries, and Gingko biloba L. | (A) Kaempferol-3-O-glucoside (B) Kaempferol-3-O-diglucoside |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Reduce serum glucose level and fasting blood glucose level Decrease the level of caspase 3 activity in β-cells Inhibit cellular apoptosis by improving anti-apoptotic Akt activities Improve cAMP signaling and insulin synthesis and secretion Improve glucose uptake by soleus muscles Reduce lipid peroxidation Decrease PPARγ expression through AMPK activity |
Rats Streptozotocin (STZ)-induced diabetic rats High fat diet mice |
Pancreatic β-cells | [90,91][85][86] | |
| 4. Quercetin | 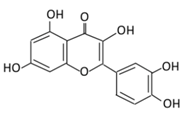 |
Black currants, cherries, apples and chokeberries | (A) Quercetin-3-O-glucoside (B) Quercetin -3-O-glucoside-7-O-glucoside (C) Quercetin-3-O-galactoside (D) Aglycone |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Inhibit insulin dependent activation of PI3K Inhibit GLUT2 which reduces the absorption of glucose in small intestine Block the activity of tyrosine kinase Improve GLUT4 translocation through the activation of AMPK Improve the recovery of cell proliferation Improve glucose absorption Reduce lipid peroxidation |
Rats Streptozotocin (STZ)-induced diabetic rats High fat diet mice |
Skeletal muscle cells Hepatocyte RINm5F β-cells |
[68,69][68][69] | |
| 5. Isorhamnetin |  |
Oenanthe javanica, Hippophae rhamnoides, and Ginkgo biloba L. | (A) Isorhamnetin (B) isorhamnetin-3-O-galacto |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Improve insulin secretion Increase glucose transporter 2 (GLUT2) Inhibit adipogenesis |
HFD- induced C57BL/6 mice | 3 T3-L1 cells | [102,103][87][88] | |
| 6. Morin | 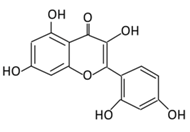 |
Psidium guajava, Prunus dulcis (Almond), chlorophora tinctoria, and fruits | (A) Morin glucuronides (B) Morin sulfates |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect. |
Reduce hepatic NF-κB activation Reduce G6Pase and FDPase enzymatic activities Increase hexokinase and G6PD enzymatic activities Improve hyperglycemia, insulin resistance, and glucose intolerance Reduce lipid peroxidation Reduce hyperlipidemia Normalize the profile of lipid and lipoprotein |
Streptozotocin (STZ)-induced diabetic rats High fructose fed rats HFD-STZ induced type 2 diabetic rats |
Rats hepatocyte | [114,115][89][90] | |
2.1.2. Rutin
2.1.3. Kaempferol
2.1.4. Isorhamnetin
2.1.5. Fisetin
2.1.6. Morin
2.2. Flavanones
2.2.1. Hesperidin
| Flavonoid Subclass | Name of Flavonoid | Structure of Flavonoid | Dietary Source | Metabolites Produced from Flavonoids | Function of Flavonoids | Mechanism of Action | Model Used | References | |
|---|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||||||
| Flavanones | 7.Hesperidin | 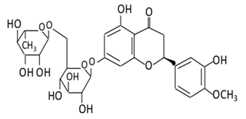 |
Orange citrus aurantium |  |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Down- regulate the production of free radical and proinflammatory cytokines Reduce oxidative stress Reduce blood glucose level by affecting glucose enzymatic activities Increase glycogen concentration and hepatic glycolysis Reduce the level of TBARS which is a byproduct of lipid peroxidation Normalize adiponectin level Increase the activity of lactate dehydrogenase (LDH) |
Alloxan-induceddiabetic rabbits Streptozotocin (STZ)-induced marginal type 1 diabetic rats (10g/kg diet) |
[122,124][122][124] | |
| 8.Naringenin | 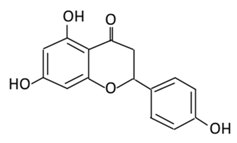 |
Grapefruit (C. paradisi), Chinese herbs like C. aurantium | Four forms could be present in the body two of them are major: (A) Naringenin glucuronides (Major form in serum) (B) Naringenin sulfates ( Major form in liver) (C) Free naringin (Not present in blood stream) D) Free naringenin (Not present in blood stream) |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Reduce poliprotein B secretion in the liver which mimic insulin effect Inhibit intestinal α-glucosidase activity which delays carbohydrates absorption | ||||
2.3.1. Apigenin
2.3.2. Luteolin
2.3.3. Tangeretin
2.3.4. Chrysin
| Flavonoid Subclass | Name of Flavonoid | Structure of Flavonoid | Dietary Source | Metabolites Produced from Flavonoids | Function of Flavonoids | Mechanism of Action | Model Used | References | |
|---|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||||||
| Flavones | 10. Baicalein | 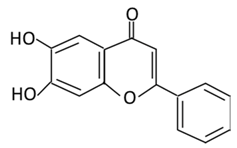 |
Scutellaria lateriflora L, and Scutellaria baicalensis Georgi | In Intestine: Baicalin will be converted into Baicalein and then absorbed rapidly. In the circulation: Baicalein will be converted to Baicalin |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Reduce the level of level of hemoglobin A1C (HbA1c) Suppress the activation of NF-κB Improve glucose tolerance and insulin secretion from pancreatic cells Improve viability of clonal β-cells which improves the production of NADH and NADPH Protect against β cells apoptosis Increase hexokinase activity in liver Activate MAPKs signaling pathway which reduce the effect of insulin resistance by phosphorylating Akt and IRS-1 and dephosphorylate NF-κB Suppress fatty acid synthesis |
Obese diabetic mice Type 2 diabetic rats |
CA1 hippocampal neurons | [172][173] |
| 11. Luteolin | 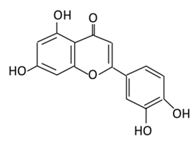 |
Parsley, broccoli, onoins leaves, celery, cabbages, apple skins, carrots, and peppers | Metabolization is medicated by UGTs and COMTs to produce: (A) Luteolin-7-glucuronide (Glucuronidated) (B) Luteolin-4-glucuronide (C) Chrysoeriol/diosmetic (Methylated) (D) Luteolin monoglucuronide (Major form in human serum | Inhibit glucose uptake by inhibiting sodium glucose co-transporter Activate AMPK pathway which increase insulin sensitivity and glucose tolerance Reduce membrane lipid peroxidation Prevent apolipoprotein B overproduction and dyslipidemia Induce hypolipidemic activity |
Streptozotocin (STZ)-induced diabetic rats High fat diet fed mice LDL receptor null mice Male Sprague-Dawley rats |
INS-1E cells | [133,135][128][129] | ||
| 9.Eriodictyol | 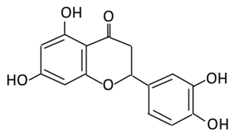 |
Lemon, Torr, Eridictyon californicum, Millettia duchesnei De Wild, and Eupatorium arnottianum | (A) Monoglucuronide M1 in the liver microsome (B) Monoglucuronide M2 in the liver microsome |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Suppress oxidative stress Decrease Intercellular Adhesion Molecule 1 (ICAM-1), Vascular endothelial growth factor (VEGF), retinal TNFα, and Endothelial NOS (eNOS). Reactivate Akt phosphorylation Reduce lipid peroxidation Up-regulate the expression of PPARγ2 Up-regulate adipocyte- specific fatty acid binding protein |
Streptozotocin induced diabetic rats (0.2%) |
HepG2 cells Differentiated 3T3-L1 cells |
[144,146][130][131] | |
2.2.2. Naringenin
2.2.3. Eriodictyol
2.3. Flavones
| (A) Antihyperglycmeic effect: | ||||||||
| (B) Hypolipemic effect | Reduce cAMP response element binding protein and histone acetyl transferase activity of CBP/p300 (NF-κB coactivator) | Reduce apoptosis Up-regulate the espression of synaptic protein which target brain cells Improve insulin secretion by supressing Maf A through NF-κB signiling pathway Activate PPAR-γ which targets adiponectin, leptin and GLUT4 genes |
Obese mice Streptozotocin induced diabetic rats Diabetic rats |
Endothelium cells Human monocytes cells |
[155][157] | |||
| 12. Diosmin | 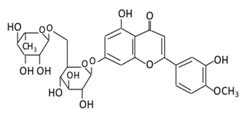 |
Citrus fruites, and Scrophularia nodosa L. | (A) Diosmin (Not excreted in urine) (B) Diosmetin (Not excreted in urine) (C) Minor metabolites in the form of glucuronic acid conjugate (Excreted in urine) |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Reduce the level of hemoglobin A1C (HbA1c) due to increase in glutathione peroxidase (GPx) Decrease G6Pase, PEPCK, and fructose-1,6-bisphosphatase enzymes Reduce plasma glucose and increase plasma insulin by activating anti-oxidant enzymes Reduce hyperglycemia by inducing β-endorphin Increase hexokinase and glucose-6-phosphate dehydrogenase activity Reduce lipid peroxidation |
Streptozotocin nicotinamide induced diabetic rats |
[174][175] |
2.5. Anthocyanins
2.5.1. Cyanidin
| Flavonoid Subclass | Name of Flavonoid | Structure of Flavonoid | Dietary Source | Metabolites Produced from Flavonoids | Function of Flavonoids | Mechanism of Action | Model used | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||||||||||
| Isoflavones | 17. Genistein | 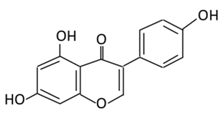 |
Soybeans, kudzu, and fava bean | 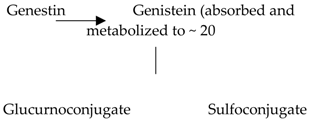 |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect |
Reduce hyperglycemia through the activity of cAMP/ PKA pathway Decrease Intercellular Adhesion Molecule 1 (ICAM-1) and p-ERK Inhibit the activity of tyrosine kinase Improve glucose intolerance and β-cells mass Decrease urinary excretion of TBARs |
Streptozotocin (STZ)-induced diabetic rats Obese diabetic mice Nongenetictype 2 diabetic mice |
INS-1 cells Human islet β-cells |
[195,199][182][186] | ||||
| 18. Daidzein | 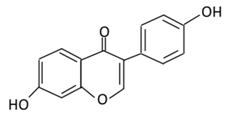 |
Soybeans, nuts, and soy milk | (A) Daidzin | (A) Antihyperglycmeic effect: | Decrease blood glucose, total cholesterol, and AMPK phosphorylation | Golden Syrian hamsters | [202,204][189][191] | ||||||
| Anthocyanins | 19. Cyanidin | 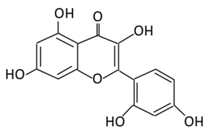 |
Bilberry, blueberry, grapes, blackberries, hawthorn, acai berry, and raspberry | (A) Anthocyanidin glucuronide conjugates (Major form in urine) (B) Simple Aglycones (Second major in urine) (C) Anthocyanidin methyl glucuronide conjugates (8 forms) (D) Cyanidin-3-glucoside E) Cyanidin-3-galactoside |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Inhibit α-glucosidase and α-amylase which reduce the absorption of glucose in small intestine Reduce fasting glucose level Prevent pancreatic apoptosis Improve antioxidant status which protects hepatocytes from HG-induced damage Attenuate aortic lipid peroxidation |
Streptozotocin (STZ)-induced diabetic rats db/db rats high fat diet fed mice |
Mouse hepatocyte | [207,209][194][196] | ||||
| 13. Apigenin | 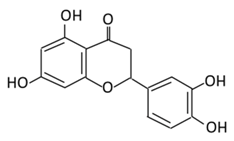 |
Onion, oranges, tea, parsley, chamomile, Hypericum perforatum L, wheat sprouts | Metabolization occurs through two phases: Phase (1): Apigenin produce three monohydroxylated: a) Luteolin b) Scutellarien c) iso-scutellarein Phase(2): Luteolin produce: a) Four monoglucuroconjugates b) Two Sulfoconjugate c) One methyl conjugate |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
|||||||||
| 20.Delphinidin | 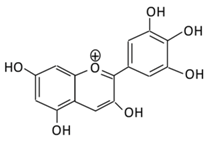 |
Dark grapes, eggplant, berries, red cabbage, carrot, and tomato | (A) 4′-O- methyl delphinidin 3-O-beta-d- glucopyranoside | Reduce cellular antioxidants | Attenuate cell damage in pancreatic β-cells Improve the morphology of the cells Improve GLUT4 translocation which lowers glucose level Increase serum cholesterol Increase lipid peroxidation |
Streptozotocin induced diabetic rats (0.2%) |
HepG2 cells Differentiated3T3-L1 cells |
(A) Antihyperglycmeic effect: | Reduce the glycation rate of HbA1c Prevents diabetes associated injuries such as endothelial cell function | [ | Diabetic mouse147 | ][ | [213149] |
| , | 215 | ] | [ | 197 | ] | [ | 198 | ] | |||||
| 21.Pelargonidin | 14.Tangeretin | 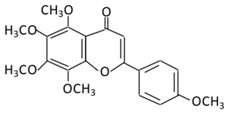 |
Poncirus trifoliate L, citrus fruit rinds, and mandarin orange | Metabolization is medicated by CYP1A1 and CYP1A2 to produce: (A) 4′ hydroxy - 5, 6, 7, 8 tetramethoxyflavone (4′-OH-TMF) |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Reduce blood glucose and HbA1c level Reduce the secretion of insulin resistance factor Increase the secretion level of insulin and insulin sensitizing factor Enhances glycolytic enzyme in the liver Reduce total cholesterol and adipocytokines level |
Rats Streptozotocin (STZ)-induced diabetic rats High fat diet mice |
Pancreatic β-cells | [160][162] | ||||
| 15. Wogonin | 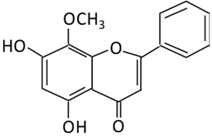 |
Scutellaria baicalensis Georgi | (A) Wogonin-7-beta-D-glucuronide (Major metabolites) (B) Wogonin-5-beta-D-glucuronide |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Reduce hyperglycemia and lipid droplets accumulation in the liver Increase vascular permeability and the expression of cell adhesion molecules Activate NF-κB and AMPK pathways Activate PPARα which has a beneficial effect on lipid metabolism |
db/db mice | 3T3-L1 cells | [176][177] | |||||
| 16. Chrysin | 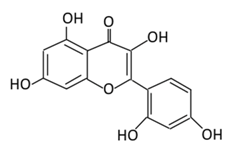 |
passiflora caerulea (L,), honey, Tilia tomentosa Moench, and Pelargonium crispum (Berg.) | (A) Chrysin glucuronides (M1) (B) Chrysin sulfates (M2) |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Reduce the level of pro-inflammatory cytokines that helps in the prevention of diabetic neuropathy Reduce blood glucose Improve renal pathology with the suppression of TGF-β, collagen-IV, and fibronectin Improve insulin level Reduce lipid peroxidation |
INS-1E cells | [167][169] | ||||||
2.4. Isoflavones
2.4.1. Genistein
2.4.2. Daidzein
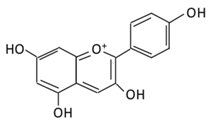 | ||||||||
| Bilberry and | ||||||||
| ficus bengalensis | ||||||||
| Linn | ||||||||
| (A) Pelargonidin- | O | -glucuronide | (B) Pelargonidin-3-galactoside |
(A) Antihyperglycmeic effect: (B) Hypolipemic effect: |
Reduce hyperglycemia Reduce the level of antioxidant defensive enzymes Stimulate insulin secretion Reduce the level of TBARS which is a byproduct of lipid peroxidation |
Streptozotocin (STZ)-induced diabetic rats Diabetic rats |
[217,219][199][200] |
3.1. Estimated Consumption Level of Flavonoids
3. Challenges Using Flavonoids
3.1. Estimated Consumption Level of Flavonoids
Flavonoids derived from vegetables and fruits are consumed in low quantities. Moreover, the content of vegetables and fruits contain not only flavonoids, but also a mixture of secondary plant metabolites. Therefore, it is difficult to stimulate this mixture into a simple purified dietary supplement [220,221][201][202]. Efforts have been made to establish an optimal human dietary consumption level of flavonoids worldwide, but the estimate methods used were poorly established [222][203]. A U.S. study on 805 men aged 65–84 years reported that the estimate intake of flavonoids from quercetin, myricetin, kaempferol, apigenin, and leuteolin was 26 mg/d and the major sources of intake were in apples, tea, and onions [223][204]. Another study conducted in the Netherlands reported a two-times higher the level of flavonoids consumed in adults compared to the U.S. study (50 mg/day) [224][205]. In addition, two Dutch studies reported the estimated consumption level of flavonoids to be 23 mg/day and 26 mg/day respectively [225,226][206][207]. These differences observed in the consumption level of flavonoids depend on dietary habits, geographical location, socioeconomic status, food processing and preparation method, solubility of flavonoids, and the ethnicity of the population. For example, in Japan, soy containing food is highly consumed and, as a result the intake of isoflavone is higher than other flavonoids subclasses [106][110]. A study reported that orange juice contains 81–200mg/L of soluble flavanones compared to 206–644 mg/L seen in the cloud which clearly suggest that processing and storage affects the concentration of flavonoids [227][208].
3.1.1. Possible Side Eects of Flavonoids Consumption
Flavonoids in bacterial and mammalian experimental studies using Ames test indicated possible genotoxicity and mutagenicity of flavonoids if consumed at higher concentrations (ranges from12.1 nmol to 225.0 nmol) [228]. Furthermore, it may alter amino acid, drug metabolism and the activity of key metabolizing enzymes [229]. Quercetin, a predominant flavonol in the human diet, showed a mutagenic eect by altering base-pair substitution and frame-shift mutation [230]. The isolated nuclei from liver rats treated with morin and naringenin showed an increase in reactive oxygen species, like hydroxyl radicals that lead to DNA degradation [231]. Additionally, flavonoids exert a cytotoxic activity as a topoisomerase II inhibitor. Genistein and quercetin are identified as topoisomerase II inhibitors, even at low concentrations (10 M), where they accumulate cleavable complexes seen in patients with secondary leukemia [232]. Genistein, naringenin, kaempferol, and daidzein were reported to inhibit thyroxine synthesis by irreversibly inhibiting thyroid peroxidation [233]. Although no data are available to state the long-termside effects of increased flavonoid consumption, following an Asian diet that contains 68 mg of flavonol and 20–240 mg of isoflavone could improve thyroid function, reduce breast cancer mortality, and should not cause adverse health effects [234].The concentrations needed for most flavonoids to generate mutagenic and cytotoxic side effects are unlikely to occur through dietary sources, but with supplementation, it could result in an increased toxic level. For instance, the recommended dosage of quercetin supplements is between 500 mg/day and 1000 mg/day, which is 20 times higher with what could be consumed in a vegetarian diet [235].
Flavonoids in bacterial and mammalian experimental studies using Ames test indicated possible genotoxicity and mutagenicity of flavonoids if consumed at higher concentrations (ranges from 12.1 nmol to 225.0 nmol) [209]. Furthermore, it may alter amino acid, drug metabolism and the activity of key metabolizing enzymes [210]. Quercetin, a predominant flavonol in the human diet, showed a mutagenic effect by altering base-pair substitution and frame-shift mutation [211]. The isolated nuclei from liver rats treated with morin and naringenin showed an increase in reactive oxygen species, like hydroxyl radicals that lead to DNA degradation [212]. Additionally, flavonoids exert a cytotoxic activity as a topoisomerase II inhibitor. Genistein and quercetin are identified as topoisomerase II inhibitors, even at low concentrations (10 μM), where they accumulate cleavable complexes seen in patients with secondary leukemia [213]. Genistein, naringenin, kaempferol, and daidzein were reported to inhibit thyroxine synthesis by irreversibly inhibiting thyroid peroxidation [214]. Although no data are available to state the long-term side effects of increased flavonoid consumption, following an Asian diet that contains 68 mg of flavonol and 20–240 mg of isoflavone could improve thyroid function, reduce breast cancer mortality, and should not cause adverse health effects [215]. The concentrations needed for most flavonoids to generate mutagenic and cytotoxic side effects are unlikely to occur through dietary sources, but with supplementation, it could result in an increased toxic level. For instance, the recommended dosage of quercetin supplements is between 500 mg/day and 1000 mg/day, which is 20 times higher with what could be consumed in a vegetarian diet [216].3.1.2. Could Flavonoid Combinations have synergistic effects?
While the amounts of flavonoids consumed is crucial to establish positive eects but also to avoid negative effects, the tables list some flavonoids that trigger multiple selected pathways improving the pathogenesis of diabetes (Figure 3, Tables 1–4). The better activity can be defined by the number of diabetes related pathways which are improved through the consumption of different flavonoids.The administration of baicalein triggers four pathways: The suppression in the NF-B pathway and fatty acid synthesis; the activation in hexokinase activity in the liver; and the protection against cell apoptosis. Quercetin prompts the activity of three dierent pathways: It improves GLUT 4 translocation; inhibits tyrosine kinase activity; and reduces lipid peroxidation. -cells apoptosis could be prevented by the administration of cyanidin or kaempferol, or baicalein. The consumption of rutin or cyanidin inhibits -glucosidase and -amylase which reduce carbohydrate absorption in the small intestine (Table 4).
While the amounts of flavonoids consumed is crucial to establish positive effects but also to avoid negative effects, the tables list some flavonoids that trigger multiple selected pathways improving the pathogenesis of diabetes (Figure 3, Table 1, Table 2, Table 3 and Table 4). The better activity can be defined by the number of diabetes related pathways which are improved through the consumption of different flavonoids. The administration of baicalein triggers four pathways: The suppression in the NF-κB pathway and fatty acid synthesis; the activation in hexokinase activity in the liver; and the protection against cell apoptosis. Quercetin prompts the activity of three different pathways: It improves GLUT 4 translocation; inhibits tyrosine kinase activity; and reduces lipid peroxidation. β-cells apoptosis could be prevented by the administration of cyanidin or kaempferol, or baicalein. The consumption of rutin or cyanidin inhibits α-glucosidase and α-amylase which reduce carbohydrate absorption in the small intestine (Table 4).Could their positive effects on diabetes be further improved by ingesting a combination of different flavonoids which complement each other by triggering additional pathways? For example, the administration of baicalein and quercetin initiates the positive eects on diabetes in six major pathways: The glucose transporter; hepatic enzymes; beta cells apoptosis; PPARs; AMPK; tyrosine kinase; and NF-B pathways. As a result of this hypothesized combination, the over activation of these pathways may be prevented, while the needed action to improve diabetes may be achieved. At this time, these are no more than suggestions which need to be proven by research. To date, little is known about flavonoids to flavonoids interactions [235]. In addition, some flavonoids showed an opposite effects on the same pathway and both lead to the improvement of diabetes. For example, fisetin has an inhibitory effects, while morin has a stimulatory effects on glucose 6 phosphate dehydrogenase and the literature states that they both improve diabetes (Figure 1). Extensive studies are required to understand the reasons behind this action—is it because of different binding sites, bioavailability, tissue exposure, absorption, or circulating concentration of these compounds. A similar pattern with different flavonoids was observed with PPAR and NF-B pathways (Tables 1–4).
Could their positive effects on diabetes be further improved by ingesting a combination of different flavonoids which complement each other by triggering additional pathways? For example, the administration of baicalein and quercetin initiates the positive effects on diabetes in six major pathways: The glucose transporter; hepatic enzymes; beta cells apoptosis; PPARs; AMPK; tyrosine kinase; and NF-κB pathways. As a result of this hypothesized combination, the over activation of these pathways may be prevented, while the needed action to improve diabetes may be achieved. At this time, these are no more than suggestions which need to be proven by research. To date, little is known about flavonoids to flavonoids interactions [216]. In addition, some flavonoids showed an opposite effect on the same pathway and both lead to the improvement of diabetes. For example, fisetin has an inhibitory effect, while morin has a stimulatory effect on glucose 6 phosphate dehydrogenase and the literature states that they both improve diabetes (Figure 1). Extensive studies are required to understand the reasons behind this action—is it because of different binding sites, bioavailability, tissue exposure, absorption, or circulating concentration of these compounds. A similar pattern with different flavonoids was observed with PPAR and NF-κB pathways (Table 1, Table 2, Table 3 and Table 4).References
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nat. Rev. Endocrinol. 2011, 8, 228–236.
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating, G. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011, 378, 31–40.
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switherland, 2006; pp. 1–50.
- Akkati, S.; Sam, K.G.; Tungha, G. Emergence of promising therapies in diabetes mellitus. J. Clin. Pharmacol. 2011, 51, 796–804.
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867.
- Reyes, J.; Tripp-Reimer, T.; Parker, E.; Muller, B.; Laroche, H. Factors Influencing Diabetes Self-Management Among Medically Underserved Patients with Type II Diabetes. Glob. Qual. Nurs. Res. 2017, 4, 2333393617713097.
- Philippe, J.; Raccah, D. Treating type 2 diabetes: How safe are current therapeutic agents? Int. J. Clin. Pract. 2009, 63, 321–332.
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551.
- Muller, L.M.; Gorter, K.J.; Hak, E.; Goudzwaard, W.L.; Schellevis, F.G.; Hoepelman, I.M.; Rutten, G.E. Increased risk of infection in patients with diabetes mellitus type 1 or 2. Ned. Tijdschr. Geneeskd. 2006, 150, 549–553.
- Pareek, H.; Sharma, S.; Khajja, B.S.; Jain, K.; Jain, G.C. Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn.). BMC Complement. Altern. Med. 2009, 9, 48.
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402.
- Skryhan, K.; Gurrieri, L.; Sparla, F.; Trost, P.; Blennow, A. Redox Regulation of Starch Metabolism. Front. Plant Sci. 2018, 9, 1344.
- Mueckler, M.; Caruso, C.; Baldwin, S.A.; Panico, M.; Blench, I.; Morris, H.R.; Lodish, H.F. Sequence and structure of a human glucose transporter. Science 1985, 229, 941–945.
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138.
- Babu, P.V.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789.
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39.
- Burgering, B.M.; Coffer, P.J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 1995, 376, 599–602.
- Dresner, A.; Laurent, D.; Marcucci, M.; Griffin, M.E.; Dufour, S.; Cline, G.W.; Shulman, G.I. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Investig. 1999, 103, 253–259.
- Schinner, S.; Scherbaum, W.A.; Bornstein, S.R.; Barthel, A. Molecular mechanisms of insulin resistance. Diabet. Med. 2005, 22, 674–682.
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem. 2000, 275, 9047–9054.
- Kile, B.T.; Schulman, B.A.; Alexander, W.S.; Nicola, N.A.; Martin, H.M.; Hilton, D.J. The SOCS box: A tale of destruction and degradation. Trends Biochem. Sci. 2002, 27, 235–241.
- Guillausseau, P.J.; Meas, T.; Virally, M.; Laloi-Michelin, M.; Medeau, V.; Kevorkian, J.P. Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes Metab. 2008, 34, S43–S48.
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22.
- Cernea, S.; Dobreanu, M. Diabetes and beta cell function: From mechanisms to evaluation and clinical implications. Biochem. Med. (Zagreb) 2013, 23, 266–280.
- Del Prato, S. Role of glucotoxicity and lipotoxicity in the pathophysiology of Type 2 diabetes mellitus and emerging treatment strategies. Diabet. Med. 2009, 26, 1185–1192.
- Szoke, E.; Gerich, J.E. Role of impaired insulin secretion and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Compr. Ther. 2005, 31, 106–112.
- Yan, L.J. Pathogenesis of chronic hyperglycemia: From reductive stress to oxidative stress. J. Diabetes Res. 2014, 137919.
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54, S97–S107.
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De novo lipogenesis in health and disease. Metabolism 2014, 63, 895–902.
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240.
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503.
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 139239.
- Moon, H.S.; Dalamaga, M.; Kim, S.Y.; Polyzos, S.A.; Hamnvik, O.P.; Magkos, F.; Mantzoros, C.S. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013, 34, 377–412.
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223.
- American Diabetes Assossiation. Standards of medical care in diabetes–2011. Diabetes Care 2011, 34, S11–S61.
- McCrimmon, R.J.; Sherwin, R.S. Hypoglycemia in type 1 diabetes. Diabetes 2010, 59, 2333–2339.
- Paulweber, B.; Valensi, P.; Lindstrom, J.; Lalic, N.M.; Greaves, C.J.; McKee, M.; Yilmaz, T. A European evidence-based guideline for the prevention of type 2 diabetes. Horm. Metab. Res. 2010, 42, S3–S36.
- Bodmer, M.; Meier, C.; Krahenbuhl, S.; Jick, S.S.; Meier, C.R. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: A nested case-control analysis. Diabetes Care 2008, 31, 2086–2091.
- Catalan, V.S.; Couture, J.A.; LeLorier, J. Predictors of persistence of use of the novel antidiabetic agent acarbose. Arch. Intern. Med. 2001, 161, 1106–1112.
- Klip, A.; Marette, A.; Dimitrakoudis, D.; Ramlal, T.; Giacca, A.; Shi, Z.Q.; Vranic, M. Effect of diabetes on glucoregulation. From glucose transporters to glucose metabolism in vivo. Diabetes Care 1992, 15, 1747–1766.
- Karnieli, E.; Armoni, M. Regulation of glucose transporters in diabetes. Horm. Res. 1990, 33, 99–104.
- Saligram, S.; Williams, E.J.; Masding, M.G. Raised liver enzymes in newly diagnosed Type 2 diabetes are associated with weight and lipids, but not glycaemic control. Indian J. Endocrinol. Metab. 2012, 16, 1012–1014.
- Forlani, G.; Di Bonito, P.; Mannucci, E.; Capaldo, B.; Genovese, S.; Orrasch, M.; Marchesini, G. Prevalence of elevated liver enzymes in Type 2 diabetes mellitus and its association with the metabolic syndrome. J. Endocrinol. Investig. 2008, 31, 146–152.
- Rangwala, S.M.; Lazar, M.A. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol. Sci. 2004, 25, 331–336.
- Krijnen, P.A.; Simsek, S.; Niessen, H.W. Apoptosis in diabetes. Apoptosis 2009, 14, 1387–1388.
- Leonidas, D.D.; Hayes, J.M.; Kato, A.; Skamnaki, V.T.; Chatzileontiadou, D.S.; Kantsadi, A.L.; Stravodimos, G.A. Phytogenic Polyphenols as Glycogen Phosphorylase Inhibitors: The Potential of Triterpenes and Flavonoids for Glycaemic Control in Type 2 Diabetes. Curr. Med. Chem. 2017, 24, 384–403.
- Ong, K.C.; Khoo, H.E. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci. 2000, 67, 1695–1705.
- Ahmad, M.; Akhtar, M.S.; Malik, T.; Gilani, A.H. Hypoglycaemic action of the flavonoid fraction of Cuminum nigrum seeds. Phytother. Res. 2000, 14, 103–106.
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356.
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Busselberg, D. Flavonoids in Cancer and Apoptosis. Cancers (Basel) 2018, 11, 28.
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S.
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S.
- Middleton, E.; Kandaswami, C., Jr.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751.
- Hossain, M.K.; Choi, H.Y.; Hwang, J.S.; Dayem, A.A.; Kim, J.H.; Kim, Y.B.; Cho, S.G. Antiviral activity of 3,4′-dihydroxyflavone on influenza a virus. J. Microbiol. 2014, 52, 521–526.
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Cho, S.G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. Int. J. Mol. Sci. 2016, 17, 569.
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. (Lond.) 2015, 12, 60.
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290.
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933.
- Barone, E.; Calabrese, V.; Mancuso, C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology 2009, 10, 97–108.
- Tanveer, A.; Akram, K.; Farooq, U.; Hayat, Z.; Shafi, A. Management of diabetic complications through fruit flavonoids as a natural remedy. Crit. Rev. Food Sci. Nutr. 2017, 57, 1411–1422.
- Del Rio, D.; Calani, L.; Scazzina, F.; Jechiu, L.; Cordero, C.; Brighenti, F. Bioavailability of catechins from ready-to-drink tea. Nutrition 2010, 26, 528–533.
- Scalbert, A.; Morand, C.; Manach, C.; Remesy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282.
- Spencer, J.P.; Schroeter, H.; Rechner, A.R.; Rice-Evans, C. Bioavailability of flavan-3-ols and procyanidins: Gastrointestinal tract influences and their relevance to bioactive forms in vivo. Antioxid. Redox Signal. 2001, 3, 1023–1039.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Kelly, G.S. Quercetin. Monograph. Altern. Med. Rev. 2011, 16, 172–194.
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89.
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282.
- Eid, H.M.; Haddad, P.S. The Antidiabetic Potential of Quercetin: Underlying Mechanisms. Curr. Med. Chem. 2017, 24, 355–364.
- Yao, Z.; Gu, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Xia, Y.; Bao, X.; Shi, H.; Sun, S.; et al. Estimated daily quercetin intake and association with the prevalence of type 2 diabetes mellitus in Chinese adults. Eur. J. Nutr. 2019, 58, 819–830.
- Fang, X.K.; Gao, J.; Zhu, D.N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008, 82, 615–622.
- Bule, M.; Abdurahman, A.; Nikfar, S.; Abdollahi, M.; Amini, M. Antidiabetic effect of quercetin: A systematic review and meta-analysis of animal studies. Food Chem. Toxicol. 2019, 125, 494–502.
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003.
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123.
- Stewart, L.K.; Wang, Z.; Ribnicky, D.; Soileau, J.L.; Cefalu, W.T.; Gettys, T.W. Failure of dietary quercetin to alter the temporal progression of insulin resistance among tissues of C57BL/6J mice during the development of diet-induced obesity. Diabetologia 2009, 52, 514–523.
- Alam, M.M.; Meerza, D.; Naseem, I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14.
- Kobori, M.; Masumoto, S.; Akimoto, Y.; Takahashi, Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin-induced disturbance of hepatic gene expression in mice. Mol. Nutr. Food Res. 2009, 53, 859–868.
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. 2003, 135C, 357–364.
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81.
- Eitah, H.E.; Maklad, Y.A.; Abdelkader, N.F.; Gamal El Din, A.A.; Badawi, M.A.; Kenawy, S.A. Modulating impacts of quercetin/sitagliptin combination on streptozotocin-induced diabetes mellitus in rats. Toxicol. Appl. Pharmacol. 2019, 365, 30–40.
- Dai, X.; Ding, Y.; Zhang, Z.; Cai, X.; Li, Y. Quercetin and quercitrin protect against cytokineinduced injuries in RINm5F beta-cells via the mitochondrial pathway and NF-kappaB signaling. Int. J. Mol. Med. 2013, 31, 265–271.
- Kreft, S.; Knapp, M.; Kreft, I. Extraction of rutin from buckwheat (Fagopyrum esculentumMoench) seeds and determination by capillary electrophoresis. J. Agric. Food Chem. 1999, 47, 4649–4652.
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312.
- Constantin, R.P.; Constantin, J.; Pagadigorria, C.L.; Ishii-Iwamoto, E.L.; Bracht, A.; Ono Mde, K.; Yamamoto, N.S. The actions of fisetin on glucose metabolism in the rat liver. Cell Biochem. Funct. 2010, 28, 149–158.
- Prasath, G.S.; Pillai, S.I.; Subramanian, S.P. Fisetin improves glucose homeostasis through the inhibition of gluconeogenic enzymes in hepatic tissues of streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2014, 740, 248–254.
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344.
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107.
- Lee, Y.S.; Lee, S.; Lee, H.S.; Kim, B.K.; Ohuchi, K.; Shin, K.H. Inhibitory effects of isorhamnetin-3-O-beta-D-glucoside from Salicornia herbacea on rat lens aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. Biol. Pharm. Bull. 2005, 28, 916–918.
- Rodriguez-Rodriguez, C.; Torres, N.; Gutierrez-Uribe, J.A.; Noriega, L.G.; Torre-Villalvazo, I.; Leal-Diaz, A.M.; Tovar, A.R. The effect of isorhamnetin glycosides extracted from Opuntia ficus-indica in a mouse model of diet induced obesity. Food Funct. 2015, 6, 805–815.
- Ricardo, K.F.S.; de Oliveira, T.T.; Jorge Nagem, T.J.; da Silva Pinto, A.; Oliveira, M.G.A.; Soares, J.F. Effect of flavonoids morin; quercetin and nicotinic acid on lipid metabolism of rats experimentally fed with triton. Braz. Arch. Biol. Technol. 2001, 44, 263–267.
- Sreedharan, V.; Venkatachalam, K.K.; Namasivayam, N. Effect of morin on tissue lipid peroxidation and antioxidant status in 1, 2-dimethylhydrazine induced experimental colon carcinogenesis. Investig. New Drugs 2009, 27, 21–30.
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102.
- Stanley Mainzen Prince, P.; Kamalakkannan, N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Mol. Toxicol. 2006, 20, 96–102.
- Niture, N.T.; Ansari, A.A.; Naik, S.R. Anti-hyperglycemic activity of rutin in streptozotocin-induced diabetic rats: An effect mediated through cytokines, antioxidants and lipid biomarkers. Indian J. Exp. Biol. 2014, 52, 720–727.
- Stanely Mainzen Prince, P.; Kannan, N.K. Protective effect of rutin on lipids, lipoproteins, lipid metabolizing enzymes and glycoproteins in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2006, 58, 1373–1383.
- Hao, H.H.; Shao, Z.M.; Tang, D.Q.; Lu, Q.; Chen, X.; Yin, X.X.; Chen, H. Preventive effects of rutin on the development of experimental diabetic nephropathy in rats. Life Sci. 2012, 91, 959–967.
- Ola, M.S.; Ahmed, M.M.; Ahmad, R.; Abuohashish, H.M.; Al-Rejaie, S.S.; Alhomida, A.S. Neuroprotective Effects of Rutin in Streptozotocin-Induced Diabetic Rat Retina. J. Mol. Neurosci. 2015, 56, 440–448.
- Jadhav, R.; Puchchakayala, G. Hypoglycemic and antidiabetic activity of flavonoids: Boswellic acid, Ellagic acid, Quercetin, Rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Int. J. Pharm. Pharm. Sci. 2012, 4, 251–256.
- An, G.; Gallegos, J.; Morris, M.E. The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab. Dispos. 2011, 39, 426–432.
- Jorge, A.P.; Horst, H.; de Sousa, E.; Pizzolatti, M.G.; Silva, F.R. Insulinomimetic effects of kaempferitrin on glycaemia and on 14C-glucose uptake in rat soleus muscle. Chem. Biol. Interact. 2004, 149, 89–96.
- Zhang, Z.; Ding, Y.; Dai, X.; Wang, J.; Li, Y. Epigallocatechin-3-gallate protects pro-inflammatory cytokine induced injuries in insulin-producing cells through the mitochondrial pathway. Eur. J. Pharmacol. 2011, 670, 311–316.
- Zanatta, L.; Rosso, A.; Folador, P.; Figueiredo, M.S.; Pizzolatti, M.G.; Leite, L.D.; Silva, F.R. Insulinomimetic effect of kaempferol 3-neohesperidoside on the rat soleus muscle. J. Nat. Prod. 2008, 71, 532–535.
- Zhang, Y.; Liu, D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur. J. Pharmacol. 2011, 670, 325–332.
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R.P.; Zhen, W.; Zhou, K.; Liu, D. The Flavonoid Kaempferol Ameliorates Streptozotocin-Induced Diabetes by Suppressing Hepatic Glucose Production. Molecules 2018, 23, 2338.
- Sharma, D.; Gondaliya, P.; Tiwari, V.; Kalia, K. Kaempferol attenuates diabetic nephropathy by inhibiting RhoA/Rho-kinase mediated inflammatory signalling. Biomed. Pharmacother. 2019, 109, 1610–1619.
- Hung, L.M.; Chen, J.K.; Huang, S.S.; Lee, R.S.; Su, M.J. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc. Res. 2000, 47, 549–555.
- Atten, M.J.; Godoy-Romero, E.; Attar, B.M.; Milson, T.; Zopel, M.; Holian, O. Resveratrol regulates cellular PKC alpha and delta to inhibit growth and induce apoptosis in gastric cancer cells. Investig. New Drugs 2005, 23, 111–119.
- Yokozawa, T.; Kim, H.Y.; Cho, E.J.; Choi, J.S.; Chung, H.Y. Antioxidant effects of isorhamnetin 3,7-di-O-beta-D-glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin-induced diabetes. J. Agric. Food Chem. 2002, 50, 5490–5495.
- Lee, J.; Jung, E.; Lee, J.; Kim, S.; Huh, S.; Kim, Y.; Park, D. Isorhamnetin represses adipogenesis in 3T3-L1 cells. Obesity (Silver Spring) 2008, 17, 226–232.
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. ARS 2013, 19, 151–162.
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000, 130, 2243–2250.
- Prasath, G.S.; Sundaram, C.S.; Subramanian, S.P. Fisetin averts oxidative stress in pancreatic tissues of streptozotocin-induced diabetic rats. Endocrine 2013, 44, 359–368.
- Kim, H.J.; Kim, S.H.; Yun, J.M. Fisetin inhibits hyperglycemia-induced proinflammatory cytokine production by epigenetic mechanisms. eCAM 2012, 639469.
- Prasath, G.S.; Subramanian, S.P. Modulatory effects of fisetin, a bioflavonoid, on hyperglycemia by attenuating the key enzymes of carbohydrate metabolism in hepatic and renal tissues in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2011, 668, 492–496.
- Althunibat, O.Y.; Al Hroob, A.M.; Abukhalil, M.H.; Germoush, M.O.; Bin-Jumah, M.; Mahmoud, A.M. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci. 2019, 221, 83–92.
- Sandireddy, R.; Yerra, V.G.; Komirishetti, P.; Areti, A.; Kumar, A. Fisetin Imparts Neuroprotection in Experimental Diabetic Neuropathy by Modulating Nrf2 and NF-κB Pathways. Cell. Mol. Neurobiol. 2016, 36, 883–892.
- Sendrayaperumal, V.; Iyyam Pillai, S.; Subramanian, S. Design, synthesis and characterization of zinc-morin, a metal flavonol complex and evaluation of its antidiabetic potential in HFD-STZ induced type 2 diabetes in rats. Chem. Biol. Interact. 2014, 219, 9–17.
- Abuohashish, H.M.; Al-Rejaie, S.S.; Al-Hosaini, K.A.; Parmar, M.Y.; Ahmed, M.M. Alleviating effects of morin against experimentally-induced diabetic osteopenia. Diabetol. Metab. Syndr. 2013, 5, 5.
- Wang, X.; Zhang, D.M.; Gu, T.T.; Ding, X.Q.; Fan, C.Y.; Zhu, Q.; Kong, L.D. Morin reduces hepatic inflammation-associated lipid accumulation in high fructose-fed rats via inhibiting sphingosine kinase 1/sphingosine 1-phosphate signaling pathway. Biochem. Pharmacol. 2013, 86, 1791–1804.
- Vanitha, P.; Uma, C.; Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Gunasekaran, P.; Ramkumar, K.M. Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and beta-cell function in streptozotocin-induced diabetic rats. Environ. Toxicol. Pharmacol. 2014, 37, 326–335.
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331.
- Visnagri, A.; Kandhare, A.D.; Chakravarty, S.; Ghosh, P.; Bodhankar, S.L. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm. Biol. 2014, 52, 814–828.
- Jung, U.J.; Lee, M.K.; Jeong, K.S.; Choi, M.S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004, 134, 2499–2503.
- Jung, U.J.; Lee, M.K.; Park, Y.B.; Kang, M.A.; Choi, M.S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int. J. Biochem. Cell Biol. 2006, 38, 1134–1145.
- Agrawal, Y.O.; Sharma, P.K.; Shrivastava, B.; Ojha, S.; Upadhya, H.M.; Arya, D.S.; Goyal, S.N. Hesperidin produces cardioprotective activity via PPAR-gamma pathway in ischemic heart disease model in diabetic rats. PLoS ONE 2014, 9, e111212.
- Akiyama, S.; Katsumata, S.; Suzuki, K.; Ishimi, Y.; Wu, J.; Uehara, M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2010, 46, 87–92.
- Akiyama, S.; Katsumata, S.; Suzuki, K.; Nakaya, Y.; Ishimi, Y.; Uehara, M. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki rats with type 2 diabetes. Biosci. Biotechnol. Biochem. 2009, 73, 2779–2782.
- Dokumacioglu, E.; Iskender, H.; Musmul, A. Effect of hesperidin treatment on α-Klotho/FGF-23 pathway in rats with experimentally-induced diabetes. Biomed. Pharmacother. 2019, 109, 1206–1210.
- Pu, P.; Gao, D.M.; Mohamed, S.; Chen, J.; Zhang, J.; Zhou, X.Y.; Jiang, H. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch. Biochem. Biophys. 2012, 518, 61–70.
- Li, J.M.; Che, C.T.; Lau, C.B.; Leung, P.S.; Cheng, C.H. Inhibition of intestinal and renal Na+-glucose cotransporter by naringenin. Int. J. Biochem. Cell Biol. 2006, 38, 985–995.
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 2012, 84, 88–92.
- Lv, P.; Yu, J.; Xu, X.; Lu, T.; Xu, F. Eriodictyol inhibits high glucose-induced oxidative stress and inflammation in retinal ganglial cells. J. Cell. Biochem. 2019, 120, 5644–5651.
- Hasanein, P.; Fazeli, F. Role of naringenin in protection against diabetic hyperalgesia and tactile allodynia in male Wistar rats. J. Physiol. Biochem. 2014, 70, 997–1006.
- Patel, K.; Singh, G.K.; Patel, D.K. A Review on Pharmacological and Analytical Aspects of Naringenin. Chin. J. Integr. Med. 2018, 24, 551–560.
- Zygmunt, K.; Faubert, B.; MacNeil, J.; Tsiani, E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem. Biophys. Res. Commun. 2010, 398, 178–183.
- van Acker, F.A.; Schouten, O.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A. Flavonoids can replace alpha-tocopherol as an antioxidant. FEBS Lett. 2000, 473, 145–148.
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits alpha-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem. Biol. Interact. 2014, 210, 77–85.
- Singh, A.K.; Raj, V.; Keshari, A.K.; Rai, A.; Kumar, P.; Rawat, A.; Maity, B.; Kumar, D.; Prakash, A.; De, A.; et al. Isolated mangiferin and naringenin exert antidiabetic effect via PPARγ/GLUT4 dual agonistic action with strong metabolic regulation. Chem. Biol. Interact. 2018, 280, 33–44.
- Choi, J.S.; Yokozawa, T.; Oura, H. Improvement of hyperglycemia and hyperlipemia in streptozotocin-diabetic rats by a methanolic extract of Prunus davidiana stems and its main component, prunin. Planta Med. 1991, 57, 208–211.
- Kannappan, S.; Anuradha, C.V. Naringenin enhances insulin-stimulated tyrosine phosphorylation and improves the cellular actions of insulin in a dietary model of metabolic syndrome. Eur. J. Nutr. 2010, 49, 101–109.
- Mulvihill, E.E.; Allister, E.M.; Sutherland, B.G.; Telford, D.E.; Sawyez, C.G.; Edwards, J.Y.; Huff, M.W. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes 2009, 58, 2198–2210.
- Annadurai, T.; Muralidharan, A.R.; Joseph, T.; Hsu, M.J.; Thomas, P.A.; Geraldine, P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin–nicotinamide-induced experimental diabetic rats. J. Physiol. Biochem. 2012, 68, 307–318.
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161.
- Zhang, W.Y.; Lee, J.J.; Kim, Y.; Kim, I.S.; Han, J.H.; Lee, S.G.; Myung, C.S. Effect of eriodictyol on glucose uptake and insulin resistance in vitro. J. Agric. Food Chem. 2012, 60, 7652–7658.
- Hameed, A.; Hafizur, R.M.; Hussain, N.; Raza, S.A.; Rehman, M.; Ashraf, S.; Ul-Haq, Z.; Khan, F.; Abbas, G. Choudhary, M.I. Eriodictyol stimulates insulin secretion through cAMP/PKA signaling pathway in mice islets. Eur. J. Pharmacol. 2018, 5, 245–255.
- Miyake, Y.; Yamamoto, K.; Tsujihara, N.; Osawa, T. Protective effects of lemon flavonoids on oxidative stress in diabetic rats. Lipids 1998, 33, 689–695.
- Shukla, S.; Fu, P.; Gupta, S. Apigenin induces apoptosis by targeting inhibitor of apoptosis proteins and Ku70-Bax interaction in prostate cancer. Apoptosis 2014, 19, 883–894.
- Panda, S.; Kar, A. Apigenin (4′,5,7-trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan-induced diabetic mice. J. Pharm. Pharmacol. 2007, 59, 1543–1548.
- Rauter, A.P.; Martins, A.; Borges, C.; Mota-Filipe, H.; Pinto, R.; Sepodes, B.; Justino, J. Antihyperglycaemic and protective effects of flavonoids on streptozotocin-induced diabetic rats. Phytother. Res. 2010, 24, S133–S138.
- Kim, E.K.; Kwon, K.B.; Song, M.Y.; Han, M.J.; Lee, J.H.; Lee, Y.R.; Park, J.W. Flavonoids protect against cytokine-induced pancreatic beta-cell damage through suppression of nuclear factor kappaB activation. Pancreas 2007, 35, e1–e9.
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Cohen, R.A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 2006, 5, 2180–2191.
- Hossain, C.M.; Ghosh, M.K.; Satapathy, B.S.; Dey, N.S.; Mukherjee, B. Apigenin causes biochemical modulation, glut4 and cd38 alterations to improve diabetes and to protect damages of some vital organs in experimental diabetes. American journal of pharmacology and toxicology. Am. J. Pharmacol. Toxicol. 2014, 9, 39–52.
- Wang, N.; Yi, W.J.; Tan, L.; Zhang, J.H.; Xu, J.; Chen, Y.; Qin, M.; Yu, S.; Guan, J.; Zhang, R. Apigenin attenuates streptozotocin-induced pancreatic β cell damage by its protective effects on cellular antioxidant defense. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 554–563.
- Malik, S.; Suchal, K.; Khan, S.I.; Bhatia, J.; Kishore, K.; Dinda, A.K.; Arya, D.S. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-κB-TNF-α and TGF-β1-MAPK-fibronectin pathways. Am. J. Physiol. Ren. Physiol. 2017, 313, F414–F422.
- Tuorkey, M.J. Molecular targets of luteolin in cancer. Eur. J. Cancer Prev. 2016, 25, 65–76.
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112.
- Ding, L.; Jin, D.; Chen, X. Luteolin enhances insulin sensitivity via activation of PPARgamma transcriptional activity in adipocytes. J. Nutr. Biochem. 2010, 21, 941–947.
- Ding, Y.; Shi, X.; Shuai, X.; Xu, Y.; Liu, Y.; Liang, X.; Su, D. Luteolin prevents uric acid-induced pancreatic beta-cell dysfunction. J. Biomed. Res. 2014, 28, 292–298.
- Zang, Y.; Igarashi, K.; Li, Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-A(y) mice. Biosci. Biotechnol. Biochem. 2016, 80, 1580–1586.
- Baek, Y.; Lee, M.N.; Wu, D.; Pae, M. Luteolin Improves Insulin Resistance in Postmenopausal Obese Mice by Altering Macrophage Polarization. Curr. Dev. Nutr. 2019, 13, FS12–FS19.
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Maugeri, A.; Calapai, G.; Gangemi, S.; Navarra, M. Chemopreventive Agents and Inhibitors of Cancer Hallmarks: May Citrus Offer New Perspectives? Nutrients 2016, 8, 698.
- Kim, M.S.; Hur, H.J.; Kwon, D.Y.; Hwang, J.T. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Mol. Cell. Endocrinol. 2012, 358, 127–134.
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine 2014, 21, 793–799.
- Miyata, Y.; Tanaka, H.; Shimada, A.; Sato, T.; Ito, A.; Yamanouchi, T.; Kosano, H. Regulation of adipocytokine secretion and adipocyte hypertrophy by polymethoxyflavonoids, nobiletin and tangeretin. Life Sci. 2011, 88, 613–618.
- Liu, Y.; Han, J.; Zhou, Z.; Li, D. Tangeretin inhibits streptozotocin-induced cell apoptosis via regulating NF-κB pathway in INS-1 cells. J. Cell. Biochem. 2019, 120, 3286–3293.
- Tran, V.H.; Duke, R.K.; Abu-Mellal, A.; Duke, C.C. Propolis with high flavonoid content collected by honey bees from Acacia paradoxa. Phytochemistry 2012, 81, 126–132.
- Mehdi, S.N.; Sana, Z.; Md, K.; Md, R.M.; Sana, Z.; Md, K.; Md, R.M. Chrysin: A Promising Anticancer Agent its Current Trends and Future Perspectives. Eur. J. Exp. Biol. 2018, 8.
- Samarghandian, S.; Azimi-Nezhad, M.; Samini, F.F.; Arkhondeh, T. Chrysin treatment improves diabetes and its complications in liver, brain, and pancreas in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2016, 94, 388–393.
- Ahad, A.; Ganai, A.A.; Mujeeb, M.; Siddiqui, W.A. Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol. Appl. Pharmacol. 2014, 279, 1–7.
- Li, R.; Zang, A.; Zhang, L.; Zhang, H.; Zhao, L.; Qi, Z.; Wang, H. Chrysin ameliorates diabetes-associated cognitive deficits in Wistar rats. Neurol. Sci. 2014, 35, 1527–1532.
- Sirovina, D.; Orsolic, N.; Koncic, M.Z.; Kovacevic, G.; Benkovic, V.; Gregorovic, G. Quercetin vs chrysin: Effect on liver histopathology in diabetic mice. Hum. Exp. Toxicol. 2013, 32, 1058–1066.
- El-Bassossy, H.M.; Abo-Warda, S.M.; Fahmy, A. Chrysin and luteolin attenuate diabetes-induced impairment in endothelial-dependent relaxation: Effect on lipid profile, AGEs and NO generation. Phytother. Res. 2013, 27, 1678–1684.
- Pu, P.; Wang, X.A.; Salim, M.; Zhu, L.H.; Wang, L.; Chen, K.J.; Li, H.L. Baicalein, a natural product, selectively activating AMPKalpha (2) and ameliorates metabolic disorder in diet-induced mice. Mol. Cell. Endocrinol. 2012, 362, 128–138.
- Yin, H.; Huang, L.; Ouyang, T.; Chen, L. Baicalein improves liver inflammation in diabetic db/db mice by regulating HMGB1/TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2018, 55, 55–62.
- Pari, L.; Srinivasan, S. Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2010, 64, 477–481.
- Jain, D.; Bansal, M.K.; Dalvi, R.; Upganlawar, A.; Somani, R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J. Integr. Med. 2014, 12, 35–41.
- Tai, M.C.; Tsang, S.Y.; Chang, L.Y.; Xue, H. Therapeutic potential of wogonin: A naturally occurring flavonoid. CNS Drug Rev. 2005, 11, 141–150.
- Ku, S.K.; Bae, J.S. Baicalin, baicalein and wogonin inhibits high glucose-induced vascular inflammation in vitro and in vivo. BMB Rep. 2015, 48, 519–524.
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043.
- Mezei, O.; Banz, W.J.; Steger, R.W.; Peluso, M.R.; Winters, T.A.; Shay, N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J. Nutr. 2003, 133, 1238–1243.
- Yang, W.; Wang, S.; Li, L.; Liang, Z.; Wang, L. Genistein reduces hyperglycemia and islet cell loss in a high-dosage manner in rats with alloxan-induced pancreatic damage. Pancreas 2011, 40, 396–402.
- Babu, P.V.; Si, H.; Fu, Z.; Zhen, W.; Liu, D. Genistein prevents hyperglycemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signaling pathway and ameliorates vascular inflammation in obese diabetic mice. J. Nutr. 2012, 142, 724–730.
- Palanisamy, N.; Viswanathan, P.; Anuradha, C.V. Effect of genistein, a soy isoflavone, on whole body insulin sensitivity and renal damage induced by a high-fructose diet. Ren. Fail. 2008, 30, 645–654.
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab. Res. Rev. 2018, 24, 74–81.
- Fu, Z.; Gilbert, E.R.; Pfeiffer, L.; Zhang, Y.; Fu, Y.; Liu, D. Genistein ameliorates hyperglycemia in a mouse model of nongenetic type 2 diabetes. Appl. Physiol. Nutr. Metab. 2012, 37, 480–488.
- Fu, Z.; Zhang, W.; Zhen, W.; Lum, H.; Nadler, J.; Bassaganya-Riera, J.; Liu, D. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 2010, 151, 3026–3037.
- Valsecchi, A.E.; Franchi, S.; Panerai, A.E.; Rossi, A.; Sacerdote, P.; Colleoni, M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur. J. Pharmacol. 2011, 650, 694–702.
- Zhou, L.; Xiao, X.; Zhang, Q.; Zheng, J.; Li, M.; Yu, M.; Wang, X.; Deng, M.; Zhai, X.; Li, R.; et al. Dietary Genistein Could Modulate Hypothalamic Circadian Entrainment, Reduce Body Weight, and Improve Glucose and Lipid Metabolism in Female Mice. Int. J. Endocrinol. 2019, 17, 2163838.
- Liggins, J.; Bluck, L.J.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein content of fruits and nuts. J. Nutr. Biochem. 2000, 11, 326–331.
- Ae Park, S.; Choi, M.S.; Cho, S.Y.; Seo, J.S.; Jung, U.J.; Kim, M.J.; Sung, M.K.; Park, Y.B.; Lee, M.K. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006, 79, 1207–1213.
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in Type 2 diabetic model mice. J. Nutr. Biochem. 2014, 25, 136–143.
- Das, D.; Sarkar, S.; Bordoloi, J.; Wann, S.B.; Kalita, J.; Manna, P. Daidzein, its effects on impaired glucose and lipid metabolism and vascular inflammation associated with type 2 diabetes. Biofactors 2018, 44, 407–417.
- Song, T.; Lee, S.O.; Murphy, P.A.; Hendrich, S. Soy protein with or without isoflavones, soy germ and soy germ extract, and daidzein lessen plasma cholesterol levels in golden Syrian hamsters. Exp. Biol. Med. (Maywood) 2003, 228, 1063–1068.
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-Anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal alpha-glucosidase and pancreatic alpha-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396.
- Nizamutdinova, I.T.; Jin, Y.C.; Chung, J.I.; Shin, S.C.; Lee, S.J.; Seo, H.G.; Kim, H.J. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol. Nutr. Food Res. 2009, 53, 1419–1429.
- Nasri, S.; Roghani, M.; Baluchnejadmojarad, T.; Rabani, T.; Balvardi, M. Vascular mechanisms of cyanidin-3-glucoside response in streptozotocin-diabetic rats. Pathophysiology 2011, 18, 273–278.
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-beta-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP-PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012, 52, 314–327.
- Gharib, A.; Faezizadeh, Z.; Godarzee, M. Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms. Planta Med. 2013, 79, 1599–1604.
- Hidalgo, J.; Teuber, S.; Morera, F.J.; Ojeda, C.; Flores, C.A.; Hidalgo, M.A.; Núñez, L.; Villalobos, C.; Burgos, R.A. Delphinidin Reduces Glucose Uptake in Mice Jejunal Tissue and Human Intestinal Cells Lines through FFA1/GPR40. Int. J. Mol. Sci. 2017, 18, 750.
- Roy, M.; Sen, S.; Chakraborti, A.S. Action of pelargonidin on hyperglycemia and oxidative damage in diabetic rats: Implication for glycation-induced hemoglobin modification. Life Sci. 2008, 82, 1102–1110.
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005, 53, 28–31.
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Ann. Rev. Nutr. 2002, 22, 19–34.
- Duthie, G.G.; Gardner, P.T.; Kyle, J.A. Plant polyphenols: Are they the new magic bullet? Proc. Nutr. Soc. 2003, 62, 599–603.
- Kuhnau, J. The flavonoids. A class of semi-essential food components: Their role in human nutrition. World Rev. Nutr. Diet. 1976, 24, 117–191.
- Sampson, L.; Rimm, E.; Hollman, P.C.; de Vries, J.H.; Katan, M.B. Flavonol and flavone intakes in US health professionals. J. Am. Diet. Assoc. 2002, 102, 1414–1420.
- Hertog, M.G.; Hollman, L.; Katan, M.B. Dietary antioxidant flavonoids of 28 vegetables and 9 fruits common consumed in the Netherlands. J. Agric. Food Chem. 1999, 40, 2379–2383.
- Arts, I.C.; Hollman, P.C.; Feskens, E.J.; Bueno de Mesquita, H.B.; Kromhout, D. Catechin intake and associated dietary and lifestyle factors in a representative sample of Dutch men and women. Eur. J. Clin. Nutr. 2001, 55, 76–81.
- Boker., K.L.; Van der Schouw, Y.T.; De Kleijn, M.J.; Jacques, P.F.; Grobbee, D.E.; Peeters, P.H. Intake of Dietary Phytoestrogens by Dutch Women. J. Nutr. 2002, 132, 1319.
- Gil Izquierdo, A.; Gil, M.; Ferreres, F.; Tomás-Barberán, F. In Vitro Availability of Flavonoids and Other Phenolics in Orange Juice. J. Agric. Food Chem. 2001, 49, 1035–1041.
- MacGregor, J.; Jurd, L. Mutagenicity of plant flavonoids: Structural requirements for mutagenic activity in Salmonella typhimurium. Mutat. Res. 1979, 54, 297–309.
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387.
- Suzuki, S.; Takada, T.; Sugawara, Y.; Muto, T.; Kominami, R. Quercetin Induces Recombinational Mutations in Cultured Cells as Detected by DNA Fingerprinting. Jpn. J. Cancer Res. Gann 1991, 82, 1061–1064.
- Skibola, C.; Smith, M. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med. 2000, 29, 375–383.
- Dassonneville, L.; Bailly, C. Chromosomal translocations and secondary leukemias induced by topoisomerase II inhibitors. Bull. Cancer 1998, 85, 254–261.
- Doerge, R.; Divi, D.L. Porphyrin πcation and protein radicals in peroxidase catalysis and inhibition by anti-thyroid chemicals. Xenobiot. Fate Foreign Compd. Biol. Syst. 1995, 25, 761–767.
- Egert, S.; Rimbach, G. Which Sources of Flavonoids: Complex Diets or Dietary Supplements? Adv. Nutr. (Bethesda Md.) 2011, 8–14.
- Sahu, S.C.; Gray, G. Interactions of flavonoids, trace metals, and oxygen: Nuclear DNA damage and lipid peroxidation induced by myricetin. Cancer Lett. 1993, 70, 73–79.
