3. In Vivo Studies of Anticancer Herbal Medicine: An Overview
The herbal medicines are tested both in vitro and in vivo. The anticancer activities of the various medicinal plants have been tested in vivo using different animal models (
Figure 3). There are many studies available on in vivo experiments of the many different anticancer plants in mice models. For instance, dihydroartemisinin was reported to inhibit tumor tissue, increase the level of interferon-gamma (IFN-γ), and decrease interleukin 4 (IL-4) in tumor-bearing mice
[60][154]. Similarly, artesunate, a derivative of artemisinin is also reported to be a promising drug against angiogenic Kaposi′s sarcoma
[61][155], growth inhibition of A549 and H1299 lung tumors by 100 mg/kg dose
[62][156], the suppression of human prostate cancer xenograft
[63][157] and the inhibition of leukemia growth in mice
[64][158].
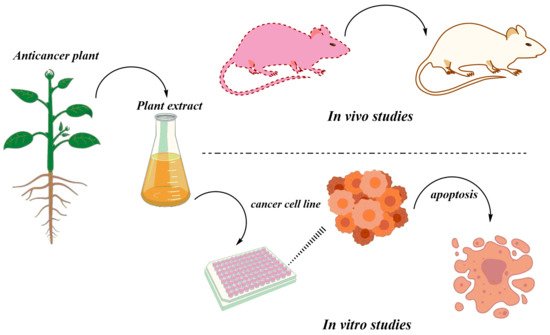
Figure 3. A depiction of general strategies applied for assaying extracts/phytochemicals from important medicinal plants for their anticancer activity both in vitro and in vivo.
Irradiation of C57BL/6 mice combined with a dose of 2 mg/kg twice a week was proved effective against lung carcinoma
[65][159]. The effectiveness of berberine was enhanced when it was used in combination with other agents. Coptisine, another alkaloid of
Coptidis rhizoma is proved to have anticancer effects when used in concentrations of 150 mg/kg against BALB/c nude mice by suppressing tumor growth and reducing cancer metastasis. The inhibition of the RAS-ERK pathway was suggested as the mechanism for this activity
[66][160]. Another study was also performed on the nude mice on the HepG2 cells by applying the aqueous extract of
H. diffusa which inhibits proliferation of cells in a dose-dependent manner, also delay S phase and arrest cells in G
0/G
1 phase
[67][161].
Similarly, a high anticancer activity of SBT-A was found in transplanted tumor nude mice. Yang et al.
[68][162] reported the anticancer activity of the polysaccharides isolated from
S. barbata by 95-D Xenograft model. The results showed that polysaccharides give strong anti-proliferative activities against a 95-D cell line. It also lowered the expression of phospho-c-Met and other signaling elements like phospho-Erk and phospho-Akt. In vivo study also gave maximum antitumor activity by using a 95-D subcutaneous xenograft model. After one daily intraperitoneal injection for 3 weeks, the tumor growth was significantly decreased (47.72 % and 13.6%) at 100 and 200 mg/kg treatments. The ex vivo studies also showed that polysaccharides of
S. barbata inhibit the phosphorylation of c-Met signaling pathway.
Furthermore, Li et al.
[69][163] isolated a steroidal saponin from
P. polyphylla which inhibited tumor growth in Lewis bearing-C57BL/6 mice and induced apoptosis in A549 cells. Results showed that steroidal saponin in concentration of 2.5, 5.0, and 7.5 mg/kg showed significant inhibition rate of 26.49 ± 17.30%, 40.32 ± 18.91%, and 54.94 ± 16.48%, remarkably increased thymus and sleep indices, decreased inflammatory cytokines (TNF-α, IL-8, and IL-10). This in turn inhibited the tumor growth in C57BL/6 mice by reduced volume and weight of tumor. Nuclear changes, DNA condensation, chromatin fragmentation, and apoptosis are induced in A549 cells with a concentration of 0.25, 0.50, and 0.75 mg/mL steroidal saponin. Tumor growth inhibited by steroidal saponin was associated with decreased ROS, inflammatory response, and induction of apoptosis.
Furthermore, Wanga et al.
[70][164] reported the effect of isoegomaketone from
P. frutescens on Huh-7 hepatoma cell carcinoma and tumor-xenograft nude mice. Results showed that isoegomaketone inhibited cells and decreased tumor weight and volume. Isoegomaketone in the concentration of 10 nM/L decreased pAkt without affecting Akt. Hepatoma cell carcinoma tumor growth was suppressed by isoegomaketone from
P. frutescens through PI3K/Akt signaling pathway blocking.
R. coptidis is also showed anticancer activity in rats as suggested by the inhibition of cyclooxygenase 2 activity. The number of aberrant crypt foci in the rat colon was decreased by 54% after the administration of
R. coptidis extracts
[71][165].
Manjamalai and Grace
[72][166] reported the apoptosis along with lowering angiogenesis and lung metastasis activities of the essential oils of
W. chinensis by using B16F-10 melanoma cell line in C57BL/6 mice. The mice were injected with B16F-10 melanoma cells through the tail vein and treated with different doses of essential oil. A 50-µg essential oil concentration showed maximum cytotoxic activities with 65.17% lethality within 24 h. The numbers of apoptotic cells increased many times in experimental samples as compared to the control group. They also recorded high levels of important proteins like p53 and caspase-3 in essential oil-treated samples compared to other non-treated samples. They recommended this plant for the treatment and control of cancer.
In vivo activities of oridonin from
R. rubescens showed a potent anticancer potential in the gallbladder
[73][113]. When injected intra-peritoneally with a concentration of 5, 10, 15 mg/kg for 3 weeks to athymic nude mice, oridonin significantly inhibited NOZ xenografts growth. Oridonin also inhibited NF-κB nuclear translocation, increased Bax/Bcl-2 ratio, activated caspase-3, caspase-9, and PARP-1 which showed that the mitochondrial pathway is concerned with apoptosis mediated by oridonin.
Studies have reported anticancer activities of two artemisinin dimer, dimer-hydrazone (dimer-Sal) and dimer-alcohol (dimer-OH) and one monomer dihydroartemisinin (DHA) compared to the control against MTLn3 breast tumors in rats. Results of the study reported that dimer-Sal, dimer-OH, and DHA significantly suppressed tumors in rats compared to the control group. It was also observed that the dimers were more potent as compared to the monomers
[74][167].
It is also reported that artemisinin is responsible for preventing breast cancer in rats treated with a single oral dose (50 mg/kg) of 7,12-dimethalbenz anthracene (DMBA) which is known for rapidly inhibiting the multiple breast tumors. After the feeding DMBA with 0–2% artemisinin to the target group and plain food in powdered form to the control group, both groups of experimental rats were monitored for breast tumors for 40 weeks. Oral artemisinin significantly reduced the development of breast tumors (57%) as compared to control fed (96%). The research indicates that artemisinin might be a potent cancer chemoprevention agent, having lesser side effects
[75][168]. Similarly, oral administration of curcumin to rats reduced the level of Gp A72 (glycoprotein) by 73% hence lowering paw inflammation
[76][169].
Tanaka et al.
[77][170] observed activities of fruit extracts of
G. indica in the azoxymethane (AOM)-induced colonic aberrant crypt foci in male model rats (F344). They found lower proliferating cell nuclear antigen index and high concentrations of glutathione S-transferase and quinone reductase. They also observed the maximum chemo-preventive activities of garcinol.
Besides mice and rats, there are many other animal models employed for studying anticancer activities. Zebrafish models are also employed for technical advantages including the ease of advanced genetic studies, expression of tumor in any organ and the striking resemblance to human malignancies
[78][171]. Zhu et al.
[79][172] used furanodiene which is a terpenoid isolated from
Rhizoma curcumae, for their anticancer effects in zebrafish models. They observed that furanodiene showed anticancer effects in a pancreatic cell line (JF 305) and human breast cancer cells (MCF-7) transplanted into zebrafish. Furanodiene showed effective results through ROS production, anti-angiogenesis, apoptosis induction, and DNA strand breaks.
Similarly, the artemisinin type compound can have anticancer activities against different types of tumors including leukemia, carcinomas of breast, kidneys, lungs, and ovaries, lymphoma, melanoma, and brain tumors
[23][80][81][23,173,174]. Currently, reports of in vivo activities of
A. annua are accumulating. One study reported anticancer activities of
A. annua against four animal models aged 10 including a male cat with malignant fibrosarcoma, a male dog with malignant mesenchymal neoplasia, a female dog with breast cancer and another male dog with a malignant fibrosarcoma. The animals were treated with various doses of 150 mg/day (3 capsules), 450 mg/day (2 capsules), 450 mg/day (3 capsules), and 450 mg/day (2 capsules). All the animals showed complete reduction with no tumor relapse
[82][175].