Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Natália Cruz-Martins.
α- and β-pinene are well-known representatives of the monoterpenes group, and are found in many plants’ essential oils. A wide range of pharmacological activities have been reported, including antibiotic resistance modulation, anticoagulant, antitumor, antimicrobial, antimalarial, antioxidant, anti-inflammatory, anti-Leishmania, and analgesic effects.
- α-pinene
- β-pinene
- pharmacological activities
- cytotoxicity
- bioavailability
- clinical studies
1. Introduction
Pinene (C10H16) is a bicyclic, double bond, terpenoid hydrocarbon [1]. α- and β-pinene are two isomers (Figure 1) found in nature, e.g., in pine (coniferous trees) essential oils (EOs). They are among the best-known representatives of a broad family of monoterpenes. α- and β-pinene enantiomers are different in their interactions with polarized light, and their mirror image does not overlap. Both structural isomers have two enantiomers (+) and (−). This difference yields four active isomers [2]. α-pinene is a colorless, water-insoluble but oil- and ethanol-soluble organic liquid. Its boiling point is 155 °C. α-pinene has been detected in at least 40 different EOs [2,3][2][3]. β-pinene is also a colorless organic liquid which is oil-soluble but ethanol- and water-insoluble. It has a boiling point ranging from 163–166 °C. It is obtained commercially by distillation or by α-pinene conversion [3]. It is also considered an essential intermediate in chilled dairy products, menthol, ionones, linalool, geraniol, citronellal, citral, citronellol, and candy production, but is mainly used in bakery products [2]. α- and β-pinene can be produced through biotransformation; some microorganisms, such as the fungi Aspergillus spp. and the bacteria Pseudomonas spp. have shown promising results in this regard. Pinenes have bicyclo [3.1.1] heptene or -heptane C-skeletons; thus, they take part in rearrangement and ring-opening reactions, producing different derivatives [4].
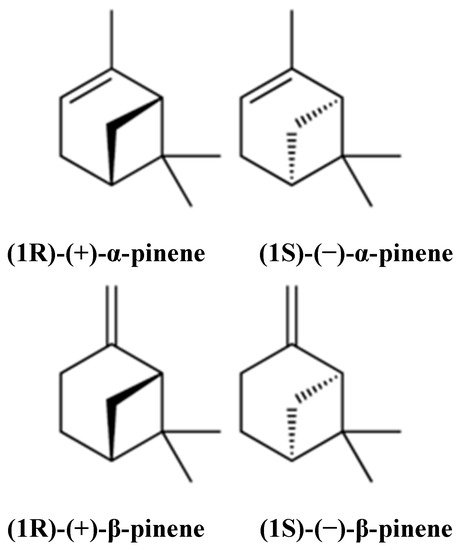
Figure 1. Chemical structures of α- and β-pinene.
These two phytochemicals exhibit diverse biological activities, leading them to various applications and uses, such as fungicidal agents, flavors, fragrances, and antiviral and antimicrobial agents [5]. In addition, α- and β-pinene are components of renal and hepatic drugs [6]. Also, α- and β-pinene are used as antibacterials due to their toxic effects on membranes [7]. Moreover, α- and β-pinene have been found to have inhibitory effects on breast cancer and leukemia [8]. The application of pinenes goes beyond natural medicine; for instance, they have been proven to be very flexible in the synthesis of polymers [9,10,11,12][9][10][11][12]; polymers synthesized from pinenes are of better quality than other polymers [13]. The safety profile of pinenes is considered outstanding, allowing their use in various chemicals, and they are generally recognized as safe (GRAS) [14]. Thus, due to their physicochemical characteristics, it is challenging in the process of biotransformation, but are still used in the production of aroma compounds [15].
Some of the plants that contain or produce α-pinene, β-pinene, or both are: Ocimum menthaefolium, Pinus spp., Juniperus communis, Rosmarinus officinalis, Lavandula stoechas, Coriandrum sativum, Cuminum cyminum, Juniperus oxycedrus, Myristica fragrans, Cinnamomum verum, Melaleuca alternifolia, Achillea millefolium, Ligusticum levisticum, Pistacia lentiscus, Grindelia camporum, Piper nigrum, Pilocarpus microphyllus, Agastache rugosa, Artemisia capillaris, Eugenia aromatic, Piper guineense, Solanum erianthum, Citrus limon, Citrus bergamia, Ferula kuhistanica, and Ferula clematidifolia [16,17,18][16][17][18].
2. Preclinical Pharmacological Activities of α- and β-Pinene
2.1. Antibiotic Resistance Modulation
Bacterial pathogens have a great ability to acquire resistance against antibiotics; a serious problem or threat for both the medical and scientific communities. A report revealed that approximately 25,000 patients die annually in Europe due to multidrug-resistant bacteria infections [19].
Gastroenteritis is a disease caused by a multidrug-resistant bacterium known as Campylobacter jejuni. A report in the U.S. declared it as a serious threat to public health [20]. α-pinene has been used as an antibiotic resistance modulator for C. jejuni [21], acting on antibacterial resistance modulation and the prevention of antimicrobial efflux (detected by the insertion mutagenesis method); this characteristic was assessed using broth microdilution and ethidium bromide accumulation assays. DNA microarrays were also used to assess the C. jejuni adaptation to α-pinene, showing that it was able to modulate the antibiotic resistance in C. jejuni considerably by reducing the MIC value of ciprofloxacin, erythromycin, and triclosan by up to 512 times. Ethidium bromide was deposited in wild-type strain at higher degrees compared to antimicrobial efflux mutant, suggesting that α-pinene targets antimicrobial efflux systems.
On the other hand, Griffiths et al. recorded the effect of α-pinene on the growth of some microbes, i.e., Nocardia sp. strain (P18.3), Pseudomona sputida PX1 (NCIB 10684), Pseudomonas sp. strain PIN18 (NCIB 10687), and P. fluorescens NCIB 11671. Strains were cultured into agar slants containing α-pinene (3 g/L in media), and their growth was analyzed. Nocardia sp. (P18.3) growth in the basal salt culture medium with α-pinene was not notable, while fast linear growth was recorded in Erlenmeyer flask cultures from vapor tubes [22]. Besides this, Pseudomonas strains (NCIB 10684, 10687, and 11,671 and PL) grew quickly when α-pinene (0.3%, v/v) was added to the growth medium.
2.2. Anticoagulative Activities
Angelica sinensis is one of the most important herbs applied in traditional Chinese medicine as crude drugs for hematopoietic and anti-inflammatory activity for healing menstrual-related diseases [23]. Yang et al. studied A. sinensis and its constituents as an anticoagulative agent. Leaves were used for administration, and New Zealand White rabbits were used as model organisms in the study. Two α-pinene derivatives were extracted from the overground parts (10 g) of A. sinensis. Thrombin time (TT) and Platelet aggregation (turbidimetric method) assays were used to assess the in vitro anticoagulative properties. Results demonstrated that α-pinene derivatives prolonged thrombin time somewhat and severely prevented platelet aggregation. These α-pinene derivatives were found to be potent anticoagulative agents, and it was stated that these compounds were able to prevent thromboxane A2 production or platelet Ca2+ promotion [24].
2.3. Antitumor Activity
Tumor is a disorder in the growth and development of cells normally categorized by excess or abnormal cells multiplication. There are two types of tumors, commonly known as benign and malignant tumors. Among all types of malignant tumor cells (cancerous cells), lung tumor is generally the most diagnosed form of tumor worldwide, causing mortality of almost 1.38 million people each year [25,26][25][26]. It is roughly classified into two types: small cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC). NSCLC is the most common and aggressive but with few limited treatment options. Survival rate of the NSCLC cases is low. Paclitaxel and carboplatin are commonly used anticancer drugs, with various side effects, such as leucopenia, hepatic and renal function disorders, nausea, and vomiting [27]. Such kinds of side effects have been minimized by coupling the drug with non-toxic enhancing agents (terpenoids with low molecular weight found in EO) and anticancer drugs [27,28,29][27][28][29]. α-pinene is an important terpenoid with anticancer properties against human ovarian cancer cell lines, hepatocellular liver carcinoma cell lines, and N2a neuroblastoma cells [30,31,32][30][31][32]. Synergistic anticancer effects have been demonstrated by α- and β-pinene against NSCLC with paclitaxel (an anticancer drug) using the combination index method and isobologram investigation [27]. A study revealed that α- and β-pinene do not exhibit considerable effects distinctly; however, when coupled with PAC, both constituents increase PAC-stimulated mitotic cell cycle arrest and apoptosis [27].
α-pinene has been shown to stimulate apoptosis proved by initial disruption of mitochondrial function, ROS formation, improved caspase-3 properties, heterochromatin aggregation, DNA disintegration, and exposure of phosphatidyl serine on the cell surface [33]. Additionally, the environment is supposed to significantly minimize cancer growth, and this was evidenced by Kusuhara et al., who stated that rats placed in fragrant environment with α-pinene exhibit a decrease in melanoma growth [34]. However, direct α-pinene did not display any in vitro effect on melanoma cell production. Chen et al. evaluated the inhibitory action of α-pinene on hepatocellular carcinoma BEL-7402 cells in vitro and in vivo using MTT assay [35,36][35][36]. α-pinene prevented BEL-7402 cells through cell cycle arrest at G2/M phase, down-regulated Cdc25C mRNA and protein expression, and decreased the action of cycle dependence on kinase 1 (CDK 1). Other studies have also suggested the use of α-pinene as a potent antitumor drug. Yang et al. measured the inhibitory properties of α-pinene on human hepatocellular carcinoma (HepG2) cells propagation, and found cell cycle arrest at G2/M phase, which seemed to be associated with down-regulation of miR-221 expression and up-regulation of CDKN1B/P27 and CDKN1C/P57 expression [37]. The effect of α-pinene on cell cycle regulation in HepG2 cells was also investigated and developed as a promising chemotherapeutic drug for administration in hepatocellular carcinoma [38]. Zhao et al. tested the inhibitory potential of α-pinene on human prostate cancer in a mouse xenograft model. β-pinene-based thiazole derivatives were used as anticancer agents via mitochondrial-facilitated apoptosis. Three human cancer cell lines, namely, cervical carcinoma HeLa cells, colon cancer CT-26, and hepatocarcinoma SMMC-7721 cells were used in the study [39]. Out of all derivatives, the compound 5g was found to inhibit cell proliferation by stimulating cell cycle arrest in HeLa cells by ROS-mediated mitochondrial dysfunction signaling pathways [40].
2.4. Genomic Instability
Catanzaro et al. studied the action of α-pinene on genomic instability in Chinese hamster cell line (V79-Cl3). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with fetal calf serum, penicillin, and streptomycin. Different doses of α-pinene (0, 25, 30, 35, 40, and 50 µM) were used to expose cells (3 × 105 per dish) for 1 h. Cytotoxicity was assessed using the standard approach. Morphological study demonstrated considerable growth in micro and multinucleated cell frequencies. Apoptotic cells were seen at 40 and 50 µM. α-pinene stimulated genetic instability, inhibiting mitotic process and leading to irregularity in 50% cells. It was established by flow cytometry that α-pinene stimulated oxidative stress and DNA destruction [41].
2.5. Cytogenetic and Oxidative Effects
Coniferous plants-derived EO are mostly composed of α-pinene. Türkez and Aydin (2013) investigated the cytogenetic and oxidative activities of α-pinene on human blood cells. Cultured human blood cells were supplemented with varying doses (0, 10, 25, 50, 75, 100, 150, and 200 mg/L) of α-pinene for 1–2 days. Lactate dehydrogenase (LDH) and MTT assays demonstrated that α-pinene at 200 mg/L reduced cell viability. Further, no changes were recorded significantly in the rates of genotoxicity endpoints. However, total antioxidant capacity (TAC) and total oxidative stress (TOS) levels revealed dose-dependent changes. TAC levels enhanced post-administration of 25 and 50 mg/L of α-pinene, while TOS levels were reduced only at 200 mg/L α-pinene on human lymphocytes [42].
2.6. Gastroprotective Effect
Gastrointestinal transit is the time it takes food to leave the stomach and travel through the intestines. It is a crucial procedure affected by many parameters that takes a long time. For healing, various medicinal plants and their compounds, such as monoterpenes (α- and β-pinene), have been used as an important source of therapeutic agents for gastrointestinal disorders [43,44][43][44]. In a study, Eucalyptus tereticornis-derived EO and its components, like α- and β-pinene, were used as gastroprotective agents [45]. Gastric emptying was measured after supplementation of a liquid test meal containing phenol red at varying time intervals. Gut was divided into consecutive segments. Small intestine transit was analyzed, and liquid test meal was supplemented. Dye retention was determined using spectrophotometric methods. Isometric contractions were observed by isometric transducers and data acquisition system. E. tereticornis EO and its components reduced gastric retention in mice, and α- and β-pinene enhanced gastric tonus in anesthetized mice. α- and β-pinene have been found to contract gastric strips in vitro, to relax the duodenum. In contrast, E. tereticornis soothes the gastric and duodenal strips. E. tereticornis speeds up the gastric emptying of liquid, and part of its effect is related to the contrast action stimulated by α- and β-pinene on gut.
The gastroprotective and antiulcerogenic properties of α-pinene were also studied using Swiss mice. Different doses (10, 30, and 100 mg/kg) of absolute ethanol and indomethacin were used to stimulate the gastric ulcer. Gastric lesions were analyzed by determining the area of lesions using the Scion Image programme. Stomach samples were crushed and sandwiched for further testing. Acute gastric lesions were introduced into the Swiss mice, and these mice fasted for 12 h. Rats were administered with 0.5 mL of vehicle (0.1% tween-80 aq. solution), ranitidine (40 mg/kg), and α-pinene (at 10–100 mg/kg), dissolved in vehicles separately. Rats were sacrificed by cervical dislocation followed by stomachs removal and scanning, with the area of lesions being determined. Gastrointestinal transit ratio was also analyzed. Findings from the study displayed that pretreatment with α-pinene cased a decline in ethanol-induced gastric mucosa lesions. α-pinene provides similar gastroprotective effects for absolute ethanol-stimulated ulcers to ranitidine (40 mg/kg) [46]. Oral pretreatment with α-pinene did not exhibit any considerable influence on indomethacin-sensitized ulcer lesions. Moreover, no considerable variation was recorded between the lesions area of α-pinene and vehicle pretreated rats. However, ranitidine decreases the area of lesions more than alternatives.
Likewise, the antiulcer properties of oleoresin (Pistacia atlantica) and α-pinene were studied on Wistar strain male albino mice [47]. Compounds were isolated from oleoresin using GC-MS. Various doses (250–2000 mg/kg b.w.) of EO were given to eight selected mice. Toxicity (restlessness, dullness, and agitation) was recorded in mice after 72 h of administration. Then, 80% ethanol was supplemented. Mice were sacrificed after 2 h, and stomachs were detached. Gastric ulcers were determined using microscope. The antibacterial properties of cultured Helicobacter pylori strains were screened using disc diffusion assay. Up to 2000 mg/kg EO was found safe. EO exhibited activity against H. pylori strains, with the inhibition zone being found maximum for the clinical strain No. 5. The EO from selected plants exhibited MIC values between 275 and 1100 μg/mL. Ethanol was used to stimulate gastric ulcers, and screening was performed against varying doses of EO and Tween 80 in water (control). Results indicated that varying doses of EO (25, 50, and 100 mg/kg) considerably reduced ethanol-induced peptic ulcers. Histopathological analysis revealed that EO reduced ethanol-induced gastric tissue damage and necrosis.
2.7. Anxiolytic-Like Effects
Yang et al. evaluated the non-rapid eye movement sleep/anxiolytic/hypnotic behavior in a rat model. Electroencephalogram (EEG) and electromyogram (EMG) analysis were used to investigate both behavior and hypnotic effect during sleep. α-pinene and zolpidem were orally pre-supplemented, followed by pentobarbital (45 mg/kg) injection. Ex vivo electrophysiological measurements from brain slices and in silico molecular modeling or molecular docking were performed. α-pinene displayed sleep-increasing behavior by direct binding to GABAA-Benzodiazepine receptors (GABAA-BZD) and forcing as a partial modulator at BZD binding site. Varying doses (12.5, 25, 50, and 100 mg/kg, orally) of α-pinene considerably reduced sleep latency and enhanced NREMS duration without any consequences on REMS and delta action. Results from the study indicated that the hypnotic action of α-pinene on rats could be due to its modulation of GABAA-BZD receptors, similar to zolpidem [48].
Similarly, the effect of α-pinene on emotional behavior, deposition, and expression of related mRNA was investigated in rat brain [49]. Rats were exposed to α-pinene (10 μL/L air), and water was used as negative control for 60/90 min. After inhalation, quantitative measurement of α-pinene in brain and gene expression by RT-PCR was performed. EPM analysis was conducted to determine the anxiolytic-like action on rats after α-pinene inhalation for 60 or 90 min. Distance was considerably enhanced when rats inhaled α-pinene for 60 min longer than the control (water). No notably variation was recorded in the total distance traveled for 90 min. α-pinene inhalation for 60 min in brain was considerably enhanced compared to 90 min. BDNF mRNA expression in olfactory bulb and hippocampus was similar at 60- and 90-min inhalation. TH mRNA expression in middle brain at 60 min inhalation was remarkably enhanced in comparison with the control.
2.8. Neuroprotective Activities
Several neurodegenerative diseases, like Alzheimer’s and Parkinson’s, are triggered by oxidative imbalance [50]. EO of various plant species are able to reduce ROS formation. Excessive ROS production led to injuries in brain functioning; thus, defense against ROS-stimulated injury is vital for appropriate brain functioning, and it diminishes the likelihood of the development of neurodegenerative disorders. With this background, Porres-Martínez et al. investigated the action of α-pinene on H2O2-sensitized oxidative stress, using mice pheochromocytoma cells (PC12) as model. Antioxidant and protective effects of α-pinene were assessed for H2O2-sensitized oxidative stress in PC12 cells. Cell viability was loosed, and cell morphology was altered after pre-treatment with α-pinene. Intracellular ROS production was prevented by α-pinene; however, it increased catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, and hemeoxygenase 1, expression. α-pinene also played an important role in reducing apoptosis; thus, it was concluded that these monoterpenes have ability to defend the nervous system [50].
2.9. Cytoprotective Activity against H2O2-Stimulated Oxidative Stress
Excessive ROS (e.g., hydroxyl radical, superoxide anion, and hydrogen peroxide) formation unbalances cellular redox, leading to oxidative stress [51] and randomly oxidizing biological molecules (i.e., peroxidation, mitochondrial dysfunction, protein carbonylation, and DNA strand breaks and base damage) [52]. ROS overproduction protection and cellular redox equilibrium may be promoted by using several natural antioxidants and EO. Salvia lavandulifolia is a species used in traditional medicine to improve memory and cure several diseases. Porres-Martínez et al. studied the constituents of S. lavandulifolia in human astrocytoma 373-MG cell line as a cytoprotective agent against H2O2-stimulated oxidative stress. Doses ranged from 10 to 250 mM α-pinene. H2O2 significantly reduces cell viability by over 60%. However, pre-supplementation with α-pinene (10, 25, 50, and 100 mM) considerably enhanced cell viability in a dose-dependent fashion (IC50 = 79.70 mM). Pre-administration with α-pinene protected U373-MG cells from H2O2-stimulated oxidative damage, through blocking the loss of cell viability (IC50: 79.70 mM to α-pinene) and cell morphology, preventing ROS formation and lipid peroxidation and enhancing the endogenous antioxidant status by enhancing glutathione, CAT, SOD, GR, and GPx activities, and HO-1 properties and protein expression [51].
2.10. Inhibitory Effect on the Growth of Endocarditis Disease
Endocarditis is a disease triggered by cardiac wall or endocardium infection, promoted by different microbes, like bacteria, fungi, and chlamydia. Microorganisms belonging to Streptococcus and Staphylococcus genus, Haemophillus parainfluenzae, H. aphrophilus, H. paraphrophilus, H. influenzae, Actinobacillus actinomycetemcomitans, Cardiobaccterium hominis, Eikenella corrodens, Kingela kingae, and K. denitrificans are the major microbial agents causing endocarditis. In modern times, microbes have shown susceptibility to drugs used in various clinical therapies. Therefore, one study was attempted to slow down the growth of endocarditis, using monoterpenes like α- and β-pinene [53]. The MIC value was determined by solid medium diffusion method, whereas viable cells count was used to investigate the interference of MIC values on bacterial cell viability. Staphylococcus aureus, S. epidermidis, S. pneumoniae, and S. pyogenes strains were utilized for the proposed screening.
Phytochemicals, like α- and β-pinene, showed inhibitory activity against all bacterial strains studied. Some phytochemicals exhibited MIC values between 5 (α-pinene x S. epidermidis SSI 1, S. pyogenes and S. pneumoniae) and 40 μL/mL (β-pinene x S. epidermidis). In addition, some bacterial strains showed resistance to antibiotics, mainly gentamicin. S. aureus exhibited resistance to α- and β-pinene.
3. Bioavailability of α-Pinene and β-Pinene
According to the Codes of Federal Regulations (FDR), Title 21; Part 314 A, bioavailability is defined as the “rate and extent to which the active ingredient or active moiety is absorbed from a drug product and becomes available at the site of drug action” [86][54]. Various studies have described that α-pinene and β-pinene display antimicrobial [87][55], anticancer [33[33][36],36], anti-inflammatory [64][56], and antiallergic [69][57] properties. Nevertheless, most of these studies lack information concerning bioavailability and pharmacokinetics of the active terpenes. However, to take advantage of the bioactivity of a particular natural product, in this case α-pinene and β-pinene, and to further use it as either a supplement or drug in future, it is necessary to study the absorption, distribution, and metabolism Monoterpenes are essentially metabolized by cytochrome P450 monooxygenases, epoxide hydrolases, and dehydrogenases to mono- and dihydroxylated substances, as well as higher oxidized metabolites that are conjugated basically to glucuronic acids [88][58]. According to the authors, after absorbing α-pinene, the proposed in vivo human metabolites are trans-verbenol (tVER), cis-verbenol (cVER), myrtenol (MYR), myrtenic acid (MYRA), αPNM3, and αPN-M1 [89][59]. In contrast, β-pinene metabolites from brushtail possum (Trichosurus vulpecula) were found to be myrtenic acid, present in their urine [90][60].3.1. Dermal Application
Kohlert, et al. [91][61] reported the preparation of eucalyptus or pine oils enriched with α- and β-pinene, camphor, 3-carene, and limonene, which have been applied in most works investigating the dermal absorption of EO-based substances, as these terpenes increase percutaneous absorption of drugs and other compounds due to their lipophilic characteristics. According to Cal and Sopala [92][62], the maximum concentration of terpenes in the stratum corneum (SC) and epidermis (ED) was obtained within 15 min of application. It was also demonstrated that the extent of absorption depends on the size of treated skin area, skin properties, concentration of administrated compound, and time of exposure [91,92][61][62].
Cal and Sopala [92][62] investigated the ex vivo skin absorption of Vicks VapoRub® (α-pinene 4.8%, β-pinene 1.1%) using human cadaver skin of a 40- to 50-year-old Caucasian women. An infinite dose of 100 mg/cm2 was applied on a 0.65 cm2 skin area; placed in a flow-through diffusion chamber; and left for 15, 30, and 60 min, respectively. For α-pinene, the maximum concentration Cmax 40 µg/cm2 in the SC was within 15 min of application of Vicks VapoRub. β-pinene reported a Cmax 290 µg/cm2 60 min after application. Although their physicochemical properties were similar, their penetration profiles into SC differed. Though the β-pinene content in the product was 4.5 times lower than that of α-pinene, the total accumulation of β-pinene in the SC was seven times greater due to its strong lipophilic nature [92][62].
β-Myrcene, limonene, α-pinene, β-pinene, linalool, geraniol, citronellol, and isomenthone are typical monoterpene constituents of rose oil [93][63]. In a study investigating the in vitro percutaneous permeation of rose oil using human tissues, Schmitt, Schaefer, Sporer and Reichling [93][63] stated that α- and β-pinene had different permeability behaviors when they were applied as a neat solution compared to as a component in rose oil (rose oil constituent). When α- and β-pinene were applied as a neat, single substance, Papp-values (apparent permeability coefficients in cm/s) were reported to be high (α-pinene 6.49 × 10−5 cm/s and β-pinene 4.48 × 10−5 cm/s) compared to other monoterpenes in rose oil applied as a neat substance. However, when pinene was available as a component of rose oil, the permeability of α-pinene dropped while the permeability of β-pinene was enhanced. The Papp-value of β-pinene was approximately four times higher (5.76 × 10−5 cm/s) than that of β-myrcene, limonene, and α-pinene (1.43 × 10−5 cm/s). It is apparent that the skin permeability was positively or negatively affected depending on the other constituents available in the mixture [93][63].
3.2. Inhalation
α- and β-pinene are insoluble in water yet soluble in blood and adipose tissues. Terpenes indicate high respiratory uptake and accumulation in adipose tissues [94][64]. In a study by Filipsson [95][65], the relative net uptake of α- and β-pinene was reported to be 62% and 66%, respectively (see Table 81).
Table 81. Bioavailability of α-pinene and β-pinene.
| Exposure | Uptake | Distribution | Elimination | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhale Air | Blood | Urine | |||||||||
| Inhalation (8 volunteers, with light exercise-50W) [81,[95,6696]][65][67] | |||||||||||
| α-pinene (+) | 2 h 450, 225, or 10 mg/m3 | Relative net uptake 59–62% * | tmax 120 min cmax 20 µMol/L(for 450 mg/m3) cmax 10 µMol/L(for 225 mg/m3) (exposure concentration depended) * cmax 10 µMol/L |
7.7% | cl21h 1.9 lkg−1h−1 |
0.001% In 30 min 4% of total uptake as cis and trans verbenol |
|||||
| - | Broth microdilution and ethidium bromide deposition | t1/2 (3 phases α, β, γ) α-4.8 min β-38 min γ-695 min |
|||||||||
| α-pinene (−) | 450 mg/m3 | 7.5% | cl21h 1.16 lkg−1h−1 |
||||||||
| Modulation of antibiotic resistance, by reducing MIC value of ciprofloxacin, erythromycin, and triclosan, up to 512 times. α-pinene also affected antimicrobial efflux systems | [ | 21 | ] | ||||||||
| 2 | - | Nocardia sp. Strain (P18.3), Pseudomonas putida PX1 (NCIB 10684), Pseudomonas sp. strain PIN18 (NCIB 10687), and P. fluorescens NCIB 11671 | - | Strains were cultured into agar slants with α-pinene (3 g/L in media), and strains growth was recorded | Nocardia sp. growth (P18.3) was not remarkable; Pseudomonas strains (NCIB 10684, 10687, and 11,671 and PL) increased promptly when α-pinene (0.3%, v/v) was added | [22] | |||||
| 24 | Citrus species | Propionibacterium acnes, Staphylococcus epidermidis | Peel EO | EO was isolated by hydrodistillation | EO demonstrated outstanding antibacterial properties against P. acnes and S. epidermidis | [68] | t1/2 α-5.6 min β-40 min γ-555 h |
||||
| 26 | Sigma-Aldrich | Escherichia coli, Micrococcus luteus, Staphylococcus aureus, and Candida albicans | - | Bioautographic method MIC was measured |
(+)-α-pinene exhibited modest activity. (−)-α-pinene was unable to display any activity. α-pinene and β-lactams revealed the highest effects. Although (−)-α-pinene revealed no positive activity, the derivatives like β-lactam, amino ester, and amino alcohol exhibited antimicrobial effects | [69] | β-pinene | 450 mg/m3 * | Relative net uptake 66% * | cmax 3 µMol/L * | * 5.7% |
| 28 | Bursera morelensis | Candida albicans strains (ATCC 14065, ATCC 32354, donated strain, and CDBB-L-1003) | Stems (EO) | EO was extracted by hydrodistillation, and GC-MS was used to isolate compounds Disc diffusion and survival curve assay were used | * cl21h 0.5 lkg−1h−1 * t |
Maximum antifungal activity was attributed to the EO and its constituent, namely, α-pinene. Minimum fungicidal concentration of EO was found to be 2 mg/mL. A slight reduction in C. albicans | 1/2 α-5.3 min β-41 min γ-25 h |
population was recorded after 12 h | Not available | ||
| [ | 70 | ] | Dermal application in vitro [93][63], Ex vivo [92][62] | ||||||||
| α-pinene | |||||||||||
| 30 | - | Staphylococcus aureus and Escherichia coli | - | Disc diffusion test, broth microdilution, and bacterial death kinetics | Inhibition halos of 11 and 12 mm for gram-positive and -negative strains were obtained at 160 µL/mL, respectively. At 1.25 and 2.5 µL/mL, (+)-α-pinene was able to eliminate bacterial colonies formation at one time of exposure of 2 h for E. coli strain | [71] | 1000 µL | ||||
| 31 | (concentration is not provided) for 27 h |
Papp 6.49 × 10−5 cm/s | |||||||||
| Syzygium cumini | Swiss mice | Leaves (EO) | MTT assay Cytotoxic effect on macrophages was determined; cells were exposed to α-pinene and tested against Leishmania |
Cytotoxic effect of α-pinene against promastigotes of Leishmania amazonensis was observed with different cell death percentages (93.7, 83.2, and 58.4%) at different concentrations (100, 50, and 25 mg/mL respectively) | [72] | 100 mg/cm2 applied on 0.65 cm2 at 37 °C ¥ | c | ||||
| 40 | max 40 µg/cm2 tmax 15 min in SC |
- | House fly (Musca domestica) | - | |||||||
| Y-tube and house flies were selected for this test | Solution with lowest concentration did not show significant differences in Y-tube arm choice. (1S)-(-)-α-pinene had maximum repellent efficiency for house flies when compared to (1R)-(+)-α-pinene | [ | 73] | β-pinene | 1000 µL (concentration is not provided) for 27 h |
Papp 4.48 × 10−5 cm/s | |||||
| 100 mg/cm2 applied on 0.65 cm2 at 37 °C ¥ | cmax 290 µg/cm2 tmax 60 min in SC |
||||||||||
| Oral administration (four volunteers) [89][59] | |||||||||||
| α-pinene | 9 mg (66 µmol) | Unmetabolized state—not detected (<4 µg/L) | t1/2 MYR-1.7 h tVER-1.0 h cVER-0.8 h |
tmax 1.6 h (metabolites) |
|||||||
| tmax 1–3 h Metabolites |
t1/2 MYR-1.5 h cVER and tVER-1.6 h MYRA-1.4 h |
||||||||||
| cmax MYR-11 µM tVER-26 µM cVER-9.3 µM |
cl24h MYR-1.5%, cVER-5.6%, tVER-4.1% MYRA-6.7%. |
||||||||||
| 78% unknown elimination, which could be exhalation or first-pass metabolism | |||||||||||
* Chamber vapour proportions α-pinene-54%, β-pinene-11%, 3-carene-35%; ¥ α-pinene 4.8%, β-pinene 1.1%, eucalyptol 3.3%, camphor 5.7%, and menthol 3.8%; trans-verbenol (tVER), cis-verbenol (cVER), myrtenol (MYR), myrtenic acid (MYRA), αPNM3, and αPN-M1, which are metabolites of α-pinene.
The mean concentration of α-pinene indicated linear kinetics with increasing exposure concentration in blood, as well as in urine [95][65]. Blood concentration mainly depends on exposure time, dose, or exposure concentration in the chamber, and the presence of other monoterpenes in the treatment chamber. Terpenes are soluble in oil; so, they are expected to accumulate in adipose tissue and thus exhibit greater tl/2 in blood. A higher fat: blood partition coefficient for α-pinene resulted in an increase in distribution rate of α-pinene from blood to fat and a higher metabolic rate for α-pinene [95][65]. During the early stages of exposure, arterial blood concentration increased rapidly and then levelled off at the end of exposure. After the end of exposure, the decaying of pinene in blood was rapid and the author distinguished three log linear phases of the pinene concentration in arterial blood, namely, initial, middle, and last. The mean half time (t1/2) of the three phases identified—initial, middle, and last—were 4.8, 38, and 695–555 min, respectively. Likewise, the respective β-pinene t1/2s were 5.3 min, 41 min, and 25 h. The long half-time of α-pinene implies that it would take more than 2 days for the body to be almost totally cleared of α-pinene (estimated as 5 × t1/2) [95][65]. Therefore, β-pinene may take more than 2 days to completely clear the body. Blood clearance values measured up to 21 h were cl21h 1.5 lkg−1h−1 for α-pinene and 0.5 lkg−1h−1 for β-pinene. Less than 0.001% of the total α-pinene dose was excreted unchanged in the urine within 0.5 h after exposure, according to Filipsson.
3.3. Oral Administration
Schmidt and Göen [89][59] recruited 4 volunteers (receiving orally α-pinene, 9 mg) and studied the metabolic products, including α-pinene, namely, myrtenol (MYR), and cis- and trans-verbenol (cVER; tVER) levels in blood and urine. The maximum blood concentration cmax for MYR was 11 µM. tVER-26µM and cVER-9.3 were recorded and metabolites concentration reached their maxima (tmax) 1.6 h after exposure. The maximum concentration of the urinary α-pinene metabolites was reached 1.6 h after exposure and then within 24 h; after it declined to pre-exposure levels with elimination half-lives of 1.5 h (MYR) and 1.6 h (cVER and tVER). The total eliminated amounts were 1.5% (MYR), 5.6% (cVER), and 4.1% (tVER) of the orally applied dose. The metabolic product, myrtenic acid (MYRA), showed renal elimination of 6.7% share of 9 mg oral dose at tmax 1.6 h after ingestion, and an elimination half-time of 1.4% [89][59]. Thus, the study showed that the blood concentration of orally administrated, unmetabolized α-pinene lasts for a short duration at low concentrations (<4 µg L−1) in blood and could be rapidly eliminated from the body unchanged via lungs. Only 22% (MYRA–7%, cVER–6%, tVER–4%, MYR–2%, αPN-M3–2%, and αPN-M1–1%) of the oral dose administrated was detected as metabolites in urine. The amount of α-pinene metabolites found in urine was notably greater compared to blood, with this being indicative of a fast transfer of metabolites from blood to urine with a rapid renal elimination. Detectable metabolite levels and non-detectable α-pinene in blood after low oral doses indicate a fast and approximately entire pre-systemic metabolism, such as hepatic or intestinal first-pass metabolism. However, it could be worthwhile to measure the other pathways of elimination, such as the composition of the exhaled air after oral administration, in order to reveal the unknown 78% (See Table 12) of the oral dose (only 22% is known as mentioned above), according to the authors [89][59].
Table 2. Antimicrobial activity of α-pinene and β-pinene.
| S. No. | Source/Species | Model | Plant Portion | Method | Result | Ref |
|---|---|---|---|---|---|---|
| α-pinene | ||||||
| 1 | Sigma Aldrich | Campylobacter jejuni | ||||
| 45 | ||||||
| Plectranthus barbatus | Malaria (Anophel es subpictus), dengue (Aedes albopictus), and Japanese encephalitis (Culex tritaeniorhynchus) mosquito vectors | EO (leaves) | GC and GC--MS were performed; larvicidal activity of EO (40, 80, 120, 160, and 200 µg/mL) and its constituents eugenol, α-pinene, and β-caryophyllene (12–100 µg/mL each) were determined by WHO methods. Mortality of larvae was measured at 24 h after exposure | EO showed substantial larvicidal effects with LC50 values of 84.20, 87.25, and 94.34 µg/mL for the selected mosquito species. For Anapheles subpictus, eugenol, α-pinene, and β-caryophyllene revealed larvicidal effects (LC50 = 25.45, 32.09, and 41.66 μg/mL), followed by Aedes albopictus (LC50 = 28.14, 34.09, and 44.77 μg/mL) and Culex tritaenior hynchus (LC50 = 30.80, 36.75, and 48.17 μg/mL, respectively) | [74] | |
| β-pinene derivatives | ||||||
| 27 | - | Klebsiella pneumoniae, Enterobacter aerogenes, S. aureus, S. epidermidis, and Candida albicans | - | 25 3-cyanopyridine compounds of β-pinene were prepared; MIC value was recorded using serial two-fold dilution method | MICs values of all derivatives ranged from 15.6 to 125 mg/l | [75] |
| 29 | - | Candida spp. | - | MIC and MFC values and microbial death curve after treatment with (+)-β-pinene enantiomers | MIC values ranged from <56.25–1800 µmol/L (+)-β-pinene. After ergosterol addition, MIC value of (+)-β-pinene was not altered, but was altered with sorbitol addition. (+)-β-pinene displayed anti-biofilm activity against multiple Candida species | [76] |
| α- and β-pinene | ||||||
| 22 | Dep. Pharmaceutical Sciences, Ponta Grossa, Brazil | Gram-positive bacteria (Staphylococcus aureus, S. epidermidis, S. pneumoniae, and S. pyogenes) | - | MIC value, viable cells count | All studied bacterial strains were sensitive to α- and β-pinene. MIC values ranged from 5 (α-pinene x S. epidermidis SSI 1; ATCC 12228; S. pyogenes ATCC 19,615; and S. pneumoniae) to 40 μL/mL (β-pinene x S. epidermidis ATCC 12228). Few bacterial strains were resistant antibiotics, mainly gentamicin. S. aureus was resistant to α- and β-pinene | [53] |
| 23 | Sigma-Aldrich | Antimicrobial: Escherichia coli (ATCC 11775, Staphylococcus aureus (ATCC 25923), Bacillus cereus (ATCC 11778), and Candida albicans (ATCC 10231). Antimalarial: Plasmodium falciparum (FCR-3) |
- | Disc diffusion method. MIC was investigated. Antimalarial properties were analyzed using the tritiated hypoxanthine incorporation assay | (+)-β-pinene was approximately two to 12 times more effective as compared to (+)-α-pinene against both gram-positive and negative bacteria, as well as C. albicans. (+)-α-pinene shows 250-fold more antimalarial activity than (+)-β-pinene | [77] |
| 25 | Sigma-Aldrich | Candida albicans, Cryptococcus neoformans, Rhizopus oryzae, and methicillin-resistant Staphylococcus aureus (MRSA) |
- | Two-fold serial dilution method was used to evaluate MIC for all the strains | MIC values of α- and β-pinene enantiomers were found to be from 117 to 6250 µg/mL. C. albicans exhibited higher sensitivity to α- and β-pinene enantiomers than MRSA. Positive enantiomers possess capability to kill 100% of C. albicans in 60 min., and 6 h was required for total killing of MRSA | [5] |
References
- Winnacker, M. Pinenes: Abundant and Renewable Building Blocks for a Variety of Sustainable Polymers. Angew. Chem. Int. Ed. 2018, 57, 14362–14371.
- Vespermann, K.A.; Paulino, B.N.; Barcelos, M.C.; Pessoa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of alpha- and beta-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817.
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin, Germany; New York, NY, USA, 2007; p. 648.
- Erman, M.B.; Kane, B.J. Chemistry around pinene and pinane: A facile synthesis of cyclobutanes and oxatricyclo-derivative of pinane from cis- and trans-pinanols. Chem. Biodivers. 2008, 5, 910–919.
- da Silva, A.C.; Lopes, P.M.; de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of alpha-pinene and beta-pinene enantiomers. Molecules 2012, 17, 6305–6316.
- Sybilska, D.; Kowalczyk, J.; Asztemborska, M.; Ochocka, R.J.; Lamparczyk, H. Chromatographic studies of the enantiomeric composition of some therapeutic compositions applied in the treatment of liver and kidney diseases. J. Chromatogr. A 1994, 665, 67–73.
- Alma, M.H.; Nitz, S.; Kollmannsberger, H.; Digrak, M.; Efe, F.T.; Yilmaz, N. Chemical composition and antimicrobial activity of the essential oils from the gum of Turkish pistachio (Pistacia vera L.). J. Agric. Food Chem. 2004, 52, 3911–3914.
- Zhou, J.Y.; Tang, F.D.; Mao, G.G.; Bian, R.L. Effect of alpha-pinene on nuclear translocation of NF-kappa B in THP-1 cells. Acta Pharmacol. Sin. 2004, 25, 480–484.
- Winnacker, M.; Rieger, B. Recent progress in sustainable polymers obtained from cyclic terpenes: Synthesis, properties, and application potential. ChemSusChem 2015, 8, 2455–2471.
- Kamigaito, M.; Satoh, K. Sustainable vinyl polymers via controlled polymerization of terpenes. In Sustainable Polymers from Biomass; Tang, C., Ryu, C.Y., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 55–90.
- Thomsett, M.R.; Moore, J.C.; Buchard, A.; Stockman, R.A.; Howdle, S.M. New renewably-sourced polyesters from limonene-derived monomers. Green Chem. 2019, 21, 149–156.
- Manfredi, K.P. Terpenes. Flavors, Fragrances, Pharmaca, Pheromones By Eberhard Breitmaier (University of Bonn). J. Nat. Prod. 2007, 70, 711.
- Satoh, K.; Nakahara, A.; Mukunoki, K.; Sugiyama, H.; Saito, H.; Kamigaito, M. Sustainable cycloolefin polymer from pine tree oil for optoelectronics material: Living cationic polymerization of β-pinene and catalytic hydrogenation of high-molecular-weight hydrogenated poly(β-pinene). Polym. Chem. 2014, 5, 3222–3230.
- Almirall, M.; Montana, J.; Escribano, E.; Obach, R.; Berrozpe, J.D. Effect of d-limonene, alpha-pinene and cineole on in vitro transdermal human skin penetration of chlorpromazine and haloperidol. Arzneim. Forsch. 1996, 46, 676–680.
- van der Werf, M.J.; de Bont, J.A.M.; Leak, D.J. Opportunities in microbial biotransformation of monoterpenes. In Biotechnology of Aroma Compounds; Berger, R.G., Babel, W., Blanch, H.W., Cooney, C.L., Enfors, S.O., Eriksson, K.E.L., Fiechter, A., Klibanov, A.M., Mattiasson, B., Primrose, S.B., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 147–177.
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70.
- Khalifaev, P.D.; Sharopov, F.S.; Bakri, M.; Habasi, M.; Safomuddin, A.; Numonov, S.; Aisa, H.A.; Setzer, W.N. Chemical composition of the essential oil from the roots of Ferula kuhistanica growing wild in Tajikistan. Nat. Prod. Commun. 2017, 12, 1–4.
- Sharopov, F.S.; Satyal, P.; Wink, M. Composition of the essential oil of Ferula clematidifolia. Chem. Nat. Compd. 2016, 52, 518–519.
- European Centre for Disease Prevention and Control (ECDC). The Bacterial Challenge: Time to React; European Center for Disease Prevention and Control EMA: Solna, Sweden, 2009; Available online: (accessed on 14 November 2019).
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States; U.S. Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013; pp. 1–114. Available online: (accessed on 14 November 2019).
- Kovac, J.; Simunovic, K.; Wu, Z.; Klancnik, A.; Bucar, F.; Zhang, Q.; Mozina, S.S. Antibiotic resistance modulation and modes of action of (-)-alpha-pinene in Campylobacter jejuni. PLoS ONE 2015, 10, e0122871.
- Griffiths, E.T.; Bociek, S.M.; Harries, P.C.; Jeffcoat, R.; Sissons, D.J.; Trudgill, P.W. Bacterial metabolism of alpha-pinene: Pathway from alpha-pinene oxide to acyclic metabolites in Nocardia sp. strain P18.3. J. Bacteriol. 1987, 169, 4972–4979.
- Nanjing University of Chinese Medicine. Dictionary of Chinese Herbal Medicines; Shanghai Science and Technology Press: Shanghai, China, 2006; p. 1207.
- Yang, N.Y.; Zhou, G.S.; Tang, Y.P.; Yan, H.; Guo, S.; Liu, P.; Duan, J.A.; Song, B.S.; He, Z.Q. Two new alpha-pinene derivatives from Angelica sinensis and their anticoagulative activities. Fitoterapia 2011, 82, 692–695.
- Alberg, A.J.; Brock, M.V.; Samet, J.M. Epidemiology of lung cancer: Looking to the future. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 3175–3185.
- American Cancer Society. Cancer Facts & Figures 2014; American Cancer Society: Atlanta, GA, USA, 2014.
- Zhang, Z.; Guo, S.; Liu, X.; Gao, X. Synergistic antitumor effect of alpha-pinene and beta-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC). Drug Res. 2015, 65, 214–218.
- Rotem, R.; Heyfets, A.; Fingrut, O.; Blickstein, D.; Shaklai, M.; Flescher, E. Jasmonates: Novel anticancer agents acting directly and selectively on human cancer cell mitochondria. Cancer Res. 2005, 65, 1984–1993.
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Carotenoids reverse multidrug resistance in cancer cells by interfering with ABC-transporters. Phytomedicine 2012, 19, 977–987.
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022.
- Cock, I.E. The phytochemistry and chemotherapeutic potential of Tasmannia lanceolata (Tasmanian pepper): A review. Pharmacogn. Commun. 2013, 3, 13–25.
- Elanur, A.; Hasan, T.; Fatime, G. Antioxidative, anticancer and genotoxicproperties of α-pinene on N2a neuroblastoma cells. Biologia 2013, 68, 1004–1009.
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Scutti, J.A.B.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454.
- Kusuhara, M.; Urakami, K.; Masuda, Y.; Zangiacomi, V.; Ishii, H.; Tai, S.; Maruyama, K.; Yamaguchi, K. Fragrant environment with alpha-pinene decreases tumor growth in mice. Biomed. Res. 2012, 33, 57–61.
- Chen, W.Q.; Xu, B.; Mao, J.W.; Wei, F.X.; Li, M.; Liu, T.; Jin, X.B.; Zhang, L.R. Inhibitory effects of alpha-pinene on hepatoma carcinoma cell proliferation. Asian Pac. J. Cancer Prev. 2014, 15, 3293–3297.
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharmacol. Sci. 2015, 127, 332–338.
- Yang, J.B.; Li, M.; Xie, J.J.; Yang, M.D.; Lu, X.S.; Wang, F.; Chen, W.Q. Effects of alpha-pinene extracted from pine needle on expression of miR-221 and its potential target genes in human hepatocellular carcinoma cells. China J. Chin. Mater. Med. 2016, 41, 3996–3999.
- Xu, Q.; Li, M.; Yang, M.; Yang, J.; Xie, J.; Lu, X.; Wang, F.; Chen, W. alpha-pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci. Rep. 2018, 38.
- Zhao, Y.; Chen, R.; Wang, Y.; Yang, Y. alpha-Pinene Inhibits Human Prostate Cancer Growth in a Mouse Xenograft Model. Chemotherapy 2018, 63, 1–7.
- Wang, Y.; Wu, C.; Zhang, Q.; Shan, Y.; Gu, W.; Wang, S. Design, synthesis and biological evaluation of novel beta-pinene-based thiazole derivatives as potential anticancer agents via mitochondrial-mediated apoptosis pathway. Bioorganic Chem. 2019, 84, 468–477.
- Catanzaro, I.; Caradonna, F.; Barbata, G.; Saverini, M.; Mauro, M.; Sciandrello, G. Genomic instability induced by alpha-pinene in Chinese hamster cell line. Mutagenesis 2012, 27, 463–469.
- Turkez, H.; Aydin, E. In vitro assessment of cytogenetic and oxidative effects of alpha-pinene. Toxicol. Ind. Health 2016, 32, 168–176.
- Schmeda-Hirschmann, G.; Yesilada, E. Traditional medicine and gastroprotective crude drugs. J. Ethnopharmacol. 2005, 100, 61–66.
- Falcao, H.S.; Mariath, I.R.; Diniz, M.F.; Batista, L.M.; Barbosa-Filho, J.M. Plants of the American continent with antiulcer activity. Phytomedicine 2008, 15, 132–146.
- Juca, D.M.; da Silva, M.T.; Junior, R.C., Jr.; de Lima, F.J.; Okoba, W.; Lahlou, S.; de Oliveira, R.B.; dos Santos, A.A.; Magalhaes, P.J. The essential oil of Eucalyptus tereticornis and its constituents, alpha- and beta-pinene, show accelerative properties on rat gastrointestinal transit. Planta Med. 2011, 77, 57–59.
- Pinheiro Mde, A.; Magalhaes, R.M.; Torres, D.M.; Cavalcante, R.C.; Mota, F.S.; Oliveira Coelho, E.M.; Moreira, H.P.; Lima, G.C.; Araujo, P.C.; Cardoso, J.H.; et al. Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis species. Pharmacogn. Mag. 2015, 11, 123–130.
- Memariani, Z.; Sharifzadeh, M.; Bozorgi, M.; Hajimahmoodi, M.; Farzaei, M.H.; Gholami, M.; Siavoshi, F.; Saniee, P. Protective effect of essential oil of Pistacia atlantica Desf. on peptic ulcer: Role of alpha-pinene. J. Tradit. Chin. Med. 2017, 37, 57–63.
- Yang, H.; Woo, J.; Pae, A.N.; Um, M.Y.; Cho, N.C.; Park, K.D.; Yoon, M.; Kim, J.; Lee, C.J.; Cho, S. alpha-Pinene, a Major Constituent of Pine Tree Oils, Enhances Non-Rapid Eye Movement Sleep in Mice through GABAA-benzodiazepine Receptors. Mol. Pharmacol. 2016, 90, 530–539.
- Kasuya, H.; Okada, N.; Kubohara, M.; Satou, T.; Masuo, Y.; Koike, K. Expression of BDNF and TH mRNA in the brain following inhaled administration of alpha-pinene. Phytother. Res. 2015, 29, 43–47.
- Porres-Martinez, M.; Gonzalez-Burgos, E.; Carretero, M.E.; Gomez-Serranillos, M.P. In vitro neuroprotective potential of the monoterpenes alpha-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. Nat. C. 2016, 71, 191–199.
- Porres-Martinez, M.; Gonzalez-Burgos, E.; Carretero, M.E.; Gomez-Serranillos, M.P. Major selected monoterpenes alpha-pinene and 1,8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharm. Biol. 2015, 53, 921–929.
- Halliwell, B. Reactive oxygen species and the central nervoussystem. J. Neurochem. 1992, 59, 1609–1623.
- Leite, A.M.; Lima, E.O.; Souza, E.L.; Diniz, M.F.; Trajano, V.N.; Medeiros, I.A. Inhibitory effect of α- and β-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Braz. J. Pharm. Sci. 2007, 43, 121–126.
- U.S. Food and Drug Administration. Electronic code of federal regulations. In Title 21 Food and Drugs; U.S. Food and Drug Administration: Washington, DC, USA, 2018.
- Köse, E.O.; Deniz, I.G.; Sarıkürkçü, C.; Aktaş, Ö.; Yavuz, M. Chemical composition, antimicrobial and antioxidant activities of the essential oils of Sideritis erythrantha Boiss. and Heldr.(var. erythrantha and var. cedretorum PH Davis) endemic in Turkey. Food Chem. Toxicol. 2010, 48, 2960–2965.
- Bae, G.-S.; Park, K.-C.; Choi, S.B.; Jo, I.-J.; Choi, M.-O.; Hong, S.-H.; Song, K.; Song, H.-J.; Park, S.-J. Protective effects of alpha-pinene in mice with cerulein-induced acute pancreatitis. Life Sci. 2012, 91, 866–871.
- Nam, S.-Y.; Chung, C.-K.; Seo, J.-H.; Rah, S.-Y.; Kim, H.-M.; Jeong, H.-J. The therapeutic efficacy of α-pinene in an experimental mouse model of allergic rhinitis. Int. Immunopharmacol. 2014, 23, 273–282.
- Schmidt, L.; Belov, V.N.; Göen, T. Sensitive monitoring of monoterpene metabolites in human urine using two-step derivatisation and positive chemical ionisation-tandem mass spectrometry. Anal. Chim. Acta 2013, 793, 26–36.
- Schmidt, L.; Göen, T. Human metabolism of α-pinene and metabolite kinetics after oral administration. Arch. Toxicol. 2017, 91, 677–687.
- Southwell, I.; Flynn, T.; Degabriele, R. Metabolism of α-and β-pinene, β-cymene and 1, 8-cineole in the brushtail possum, Trichosurus vulpecula. Xenobiotica 1980, 10, 17–23.
- Kohlert, C.; Van Rensen, I.; März, R.; Schindler, G.; Graefe, E.; Veit, M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med. 2000, 66, 495–505.
- Cal, K.; Sopala, M. Ex vivo skin absorption of terpenes from Vicks VapoRub ointment. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2008, 14, Pi19–Pi23.
- Schmitt, S.; Schaefer, U.; Sporer, F.; Reichling, J. Comparative study on the in vitro human skin permeation of monoterpenes and phenylpropanoids applied in rose oil and in form of neat single compounds. Die Pharm. 2010, 65, 102–105.
- Falk, A.; Gullstrand, E.; Löf, A.; Wigaeus-Hjelm, E. Liquid/air partition coefficients of four terpenes. Occup. Environ. Med. 1990, 47, 62–64.
- Filipsson, A.F. Short term inhalation exposure to turpentine: Toxicokinetics and acute effects in men. Occup. Environ. Med. 1996, 53, 100–105.
- Falk, A.A.; Hagberg, M.T.; Löf, A.; Wigaeus-Hjelm, E.; Zhiping, W. Uptake, distribution and elimination of a-pinene in man after exposure by inhalation. Scand. J. Work Env. Health 1990, 16, 372–378.
- Levin, J.-O.; Eriksson, K.; Falk, A.; Löf, A. Renal elimination of verbenols in man following experimental α-pinene inhalation exposure. Int. Arch. Occup. Environ. Health 1992, 63, 571–573.
- Baik, J.S.; Kim, S.S.; Lee, J.A.; Oh, T.H.; Kim, J.Y.; Lee, N.H.; Hyun, C.G. Chemical composition and biological activities of essential oils extracted from Korean endemic citrus species. J. Microbiol. Biotechnol. 2008, 18, 74–79.
- Dhar, P.; Chan, P.; Cohen, D.T.; Khawam, F.; Gibbons, S.; Snyder-Leiby, T.; Dickstein, E.; Rai, P.K.; Watal, G. Synthesis, antimicrobial evaluation, and structure-activity relationship of alpha-pinene derivatives. J. Agric. Food Chem. 2014, 62, 3548–3552.
- Rivera-Yanez, C.R.; Terrazas, L.I.; Jimenez-Estrada, M.; Campos, J.E.; Flores-Ortiz, C.M.; Hernandez, L.B.; Cruz-Sanchez, T.; Garrido-Farina, G.I.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, alpha-Pinene and gamma-Terpinene-An In Vitro Study. Molecules 2017, 22, 2095.
- de Sousa Eduardo, L.; Farias, T.C.; Ferreira, S.B.; Ferreira, P.B.; Lima, Z.N.; Ferreira, S.B. Antibacterial Activity and Time-kill Kinetics of Positive Enantiomer of alpha-pinene Against Strains of Staphylococcus aureus and Escherichia coli. Curr. Top. Med. Chem. 2018, 18, 917–924.
- Rodrigues, K.A.; Amorim, L.V.; Dias, C.N.; Moraes, D.F.; Carneiro, S.M.; Carvalho, F.A. Syzygium cumini (L.) Skeels essential oil and its major constituent alpha-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J. Ethnopharmacol. 2015, 160, 32–40.
- Haselton, A.T.; Acevedo, A.; Kuruvilla, J.; Werner, E.; Kiernan, J.; Dhar, P. Repellency of alpha-pinene against the house fly, Musca domestica. Phytochemistry 2015, 117, 469–475.
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Bhattacharyya, A.; Benelli, G. Eugenol, alpha-pinene and beta-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res. 2016, 115, 807–815.
- Liao, S.; Shang, S.; Shen, M.; Rao, X.; Si, H.; Song, J.; Song, Z. One-pot synthesis and antimicrobial evaluation of novel 3-cyanopyridine derivatives of (-)-beta-pinene. Bioorg. Med. Chem. Lett. 2016, 26, 1512–1515.
- de Macedo Andrade, A.C.; Rosalen, P.L.; Freires, I.A.; Scotti, L.; Scotti, M.T.; Aquino, S.G.; de Castro, R.D. Antifungal Activity, Mode of Action, Docking Prediction and Anti-biofilm Effects of (+)-beta-pinene Enantiomers against Candida spp. Curr. Top. Med. Chem. 2018, 18, 2481–2490.
- van Zyl, R.L.; Seatlholo, S.T.; van Vuuren, S.F. The biological activities of 20 nature identical essential oil constituents. J. Essent Oil Res. 2006, 18, 129–133.
More
