This entry highlights systematic progress in the design of synthetic glycolipid (neoglycolipids) analogs evolving from the conventional architectures of natural glycosphingolipids and gangliosides. Given that naturally occurring glycolipids are composed of only one hydrophilic sugar head-group and two hydrophobic lipid tails embedded in the lipid bilayers of the cell membranes, they usually require extraneous lipids (phosphatidylcholine, cholesterol) to confer their stability. In order to obviate the necessity for these additional stabilizing ingredients, recent investigations have merged dendrimer chemistry with that of neoglycolipid syntheses.
- glycolipids
- neoglycolipids
- dendrimers
- liposomes
- dendrimersomes
- carbohydrates
1. Introduction
A large number of classical therapeutic drugs have limited clinical efficacy due to their constrained capacity to reach the targeted tissues or because they are linked to harmful toxic effects at the large doses required to compensate for these weaknesses. Drug delivery through encapsulation into liposomes has been a real breakthrough in improving the therapeutic index of several drugs, particularly in cancer where liposomes were first applied [1]. Liposomes are nanometer-size nanoparticles, often spherical, capable of incorporating either hydrophobic or hydrophilic molecules within the lipid bilayer or the aqueous cavity, respectively. Several hundreds of drugs, including anticancer and antimicrobial agents, chelating agents, peptide hormones, enzymes, proteins, vaccines, and genetic materials, have been encapsulated into the aqueous or lipid phases of liposomes. One of the most definitive demonstrations of these excellent properties has recently been widely observed with the SARS-CoV-2 vaccines of Pfizer-BioNTech and Moderna encapsulating mRNAs. Liposome technologies have greatly matured and they are now formulated into various sizes, compositions, and other characteristics, including surface groups anchoring capable of specific tissue targeting [1][2][3][1,2,3], such as carbohydrates [4]. In this way, they can selectively deliver to the target site for in vivo applications, thereby, dramatically increasing the therapeutic index of otherwise deleterious drugs
However, aqueous solutions of liposomes face physical and chemical instabilities during long-term storage. Hydrolysis and oxidation of phospholipids and liposome aggregations are the most common cause of their instabilities. Interestingly, carbohydrates have been investigated for their ability to protect liposomes against fusion and leakage during lyophilization processes. A particular aspect has been the discovery that sugars offer cryo-protection [1]. Recent liposomal formulations, such as PEGylated liposomes (stealth-liposomes), can extend blood circulation time and vary drug distribution in the body, which can also reduce possible cardiotoxicity. Several lipid structures are commonly found in modern liposome formulations ( Figure 1 ).
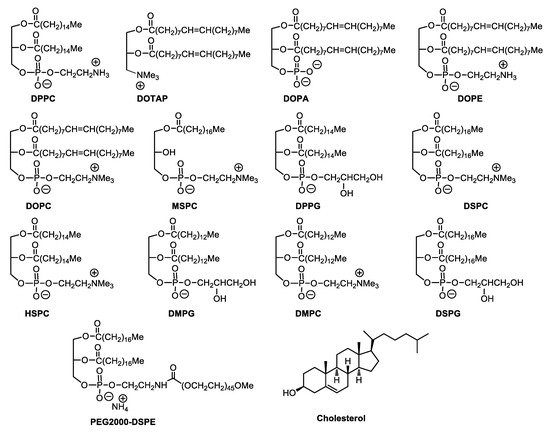
2. Natural Glycolipids
Glycolipids are important members of the glycoconjugate family [4]. They are endowed with natural amphiphilicity since they are composed of hydrophilic carbohydrate head groups and lipophilic tails. They are essential molecules amongst biomolecules as they are implicated in many complex biological processes. Several simple representatives are also used as surfactants in detergency or emulsification technology. In the complexity of biological interactions and cell-cell communications, their amphiphilicity is mostly responsible for their physicochemical activities and peculiar functions [5]. For glycolipids of biological relevance, this is associated with their location in, out or within cell membranes and their aptitude to cross it. More importantly, they can associate together on the cell surfaces to form colonies forming rafts that are responsible of fundamental multivalent carbohydrate-protein and carbohydrate-carbohydrate interactions [6][7][8][9][10][11][12][13][14][15][6,7,8,9,10,11,12,13,14,15] having physiological significances [16].
Glycolipid-forming liposomes closely resemble that of cell membranes, albeit greatly simplified. They have been comprehensively studied as models of cell membranes [4]. Reconstitution of the membrane-bound carbohydrates within liposome bilayers has been one of the most useful techniques in studying the functions of the membrane glycoconjugates. Glycoliposomes are attractive as delivery systems due to their capacity to improve the stability, therapeutic efficiency, and pharmacokinetic properties of drugs while reducing their side effects and have the advantage of being biodegradable and nontoxic. In addition, surface chemistry and lipid composition can be easily modified to address their precise applications. Cell surface carbohydrates have specific interactions with their cognate proteins, which play an important role in various biological recognition processes, such as fertilization, metastasis, inflammations, and host–pathogen adhesion. Therefore, they serve as attractive molecules for surface modification of liposomes with purpose for specific biomedical applications.
Glycolipids can be classified based on their lipid moieties as glycoglycerolipids, glycophosphatidylinositols, and glycosphingolipids. Glycolipids are found in membranes of most living organisms and their carbohydrate components are directly involved with their recognition/protection properties [17][18][17,18]. Microbial-derived glycolipids are increasingly serving as models for sustainable and stable sources of highly diversified, yet simple and economically viable glycolipids [19]. Together with other glycoconjugate members of cell surface glycoproteins, they form the glycocalyx, which coats the cells that provide contact with their environment. Carbohydrates and their cognate glycolipids and glycoproteins at the cell surfaces are involved in key biological phenomena, such as tumor cell expression or markers, bacterial and viral infections, inflammatory responses, and immune cell regulations. The precise role of the lipid tails of glycolipids, embedded within the lipid bilayers of the cells, is not yet fully understood. Nonetheless, they play a major role in correctly presenting carbohydrate antigens to other cells or to protein receptors, as well as in membrane stability and overall organization. Although the lipid components of eukaryotic glycolipids are mostly built around sphingolipids, those of prokaryotic organisms are much more diversified and complex.
Analysis of the self-assembling properties of glycolipid analogues has highlighted the crucial role of the conformations and molecular packing of the hydrophobic chains in the understanding of the interfacial aggregation phenomena. The physicochemical properties of artificial lipid architectures, mimicking the natural membrane composition, are informative for their exploitation in designing more stable and viable version of biologically relevant vesicles [20] and for drug delivery [21]. Therefore, optimizing the molecular architectures of glycolipids carbohydrate has been the subject of intense activities [22]. The high polarity and biological recognition patterns of the carbohydrate moieties coupled with the hydrophobic character of the lipid tails are both intimately related and make these two molecular entities responsible for the nanomaterial properties they are endowed with.
3. Arborescent Neoglycolipids as Detergents
The study of integral membrane-bound protein structures and functions is troublesome due to the difficulty in extracting and handling these highly lipophilic proteins. Classical examples are the family of G protein coupled receptors (GPCRs). Aqueous solubilization, necessary for common biophysical analysis (ex. NMR), generally requires a detergent to shield the large lipophilic surfaces of the native proteins. Detergents are valuable tools for membrane protein manipulation. The micellar aggregates formed by detergent have the ability to encapsulate the hydrophobic domains of membrane proteins. The resulting protein−detergent complexes become compatible with the aqueous environments, making structural and functional analyses feasible.
Many proteins remained difficult to investigate due to the lack of suitable detergents. Chae and coworkers have introduced a class of arborescent amphiphiles with hydrophilic groups derived from maltose ( 77, 78) ( Scheme 12 ) [23][44]. Representatives of this maltose–neopentyl glycol (MNG) amphiphile family showed favorable behavior relative to conventional detergents, as manifested in multiple membrane protein systems, leading to enhanced structural stability and successful crystallization. Therefore, MNG neoglycoamphiphiles represent promising tools for the structural analyses of membrane-bound protein. Moreover, they recently also disclosed improve families of these interesting detergents as exemplified by the mannitol-based amphiphilic compound 79 [24][45] and the highly branched pentasaccharide 80 [25][46] ( Scheme 11 ). The authors rationalized that the protein-detergent complexes formed were smaller with these novel arborescent neoglyco- amphiphiles. Several members of these families of detergents are commercially available [26][47].
4. Arborescent Neoglycolipids Built from Cyclic Scaffolds
Other valuable approaches to self-assembling amphiphilic and arborescent glycoarchitectures based on cyclic scaffolds such as calix[4]resorcarenes and cyclodextrins have provided interesting nanomaterials. For instance, a macrocyclic glycoconjugate having four hydrophobic undecyl chains and eight oligosaccharide moieties (oligomaltose) on the opposite sides of a calix[4]resorcarene macrocycle ( 83) has been described by the Aoyama’s group [27][48]. It has been prepared from a range of maltooligosaccharide lactones ( 81) and an octaamino derivative of the calix[4]resorcarene ( 82) ( Scheme 13 ). The authors showed that they form small micelle-like nanoparticles (d = 3 nm) in water based on dynamic light scattering (DLS), gel permeation chromatography (GPC), and transmission electron microscopy (TEM). Curiously, the micellar nanoparticles agglutinated in the presence of Na 2HPO 4/ NaH 2PO 4 forming aggregates up to 60–100 nm, as revealed by DLS as well as microscopy (TEM and AFM). The phosphate-induced agglutination processes could be followed by surface plasmon resonance (SPR). Kinetic analyses demonstrated that the phosphate-mediated inter(saccharide) interactions were significantly dependent on the oligosaccharide chain lengths (n), becoming more favorable with increasing n’s.
The attachment of biologically relevant carbohydrate head groups by covalent bonding in several copies and at exact positions of cyclomaltooligosaccharides (cyclodextrins, CDs) has been a highly productive and flexible strategy for the syntheses of multivalent glyconanomaterials [28][49]. The commercial availability of CDs in three different sizes (α-, β-, and γ-CDs) combined with their hydroxyl groups of varied accessibilities and reactivity allow excellent control of their regiochemical functionalization. ‘ ‘Click-type’’ ligation chemistries, including copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC), thiol–ene coupling or thiourea-forming reactions, have been systematically fulfilled to secure full homogeneity of the resulting glycoconjugates. CD-based glycoconjugates constitute [28][29][30][49,50,51] key players in studying and understanding the fundamental structural features deciphering multivalent carbohydrate-protein recognition events [6][7][8][9][10][11][12][13][14][15][6,7,8,9,10,11,12,13,14,15]. The approach has also been applied using chemoenzymatic glycan synthesis [31][52]. Nanometric glycoarchitectures, endowed with the flexibility of adapting the nature and inter-saccharide distances and orientations in the presence of their cognate receptors, such as lectins or capable of mimicking the fluidity of biological membranes, have been particularly well-adapted by self-assembling amphiphilic glycans. In addition, the role played by glyconanomaterials nicely positions them toward applications in cancer therapies [32][33][53,54]. Moreover, such well-defined glycoconjugates are useful for deepening our understanding of the sugar code [34][55].
Amphiphilic 7-membered β-cyclodextrins with alkylthio chains at the primary-hydroxyl side and galactosylthio-oligo-(ethylene glycol) units at the most reactive secondary-hydroxyl groups, pointing the bottom segment of the cone-shaped CD, have been clear examples of the role played by a proper balancing of the hydrophilic/hydrophobic partners ( Scheme 14 ) [35][36][56,57]. These molecules formed nanoparticles and vesicles having strong multivalent effects in their binding to the bacterial lectin PA-1L from Pseudomonas aeruginosa . The balance between hydrophobic and hydrophilic components in amphiphilic β-cyclodextrins, targeted by receptor specific glucoside or galactoside groups possessing either hexyl ( 84) or hexadecyl ( 85) alkyl chains have been shown to dramatically influence the structural properties of these systems. The dissimilar amphiphilic features of single cyclodextrins generated micellar aggregates and vesicles with an internal aqueous compartment able to encapsulate guests. Small-angle light scattering (SAXS) , cryo-TEM and AFM investigations describe the size and shape of these self-assembling structures. Selective binding interactions with the carbohydrate moieties of the nanoassemblies by a PA-1L lectin with 85 has been successfully demonstrated using time-resolved fluorescence.
An exhaustive review on amphiphilic β-cyclodextrins harboring arborescent architectures have been described by the group of García Fernández et al. [37][58]. Moreover, analogous constructs were recently described using doxorubicin-loaded (Dox) glycodendrimersomes armed with mannopyranoside dendrons that are used as targeting components. They have been efficiently prepared from per-6-azido-β-cyclodextrin [38][59]. The amphiphilic neoglycolipids were synthesized using short propionic or valeric anhydrides at the secondary hydroxyl groups, while propargyl α-D-mannopyranoside was once again appended by azide–alkyne cycloaddition (CuAAC). Once loaded with Dox, the resulting Dox-glyconanomaterials were efficiently taken up via receptor-mediated endocytosis by MDA-MB-231 breast cancer cells that overexpress the mannose receptor. After cellular uptake, the low intracellular pH caused the release of DOX, which triggered apoptosis. Based on dynamic light scattering (DLS) measurements, the propionic anhydride-modified self-assembled formats formed nanoparticles with an average hydrodynamic size of 112 nm (PDI, 0.109) that increased to 199 nm (PDI, 0.141) after Dox-inclusion. The particle size and morphology of the liposomes were evaluated using transmission electron microscopy (TEM), which showed nanoparticles having spherical shapes with an average diameter of 45 nm. The same materials incorporating both doxorubicin and amphotericin B were used to target the delivery of the combined drugs into macrophage cells. The combination of both drugs lead to enhanced anti-leishmanial therapeutic efficacy through a synergistic effect [39][60].
