Tetrodotoxin (TTX) is a potent neurotoxin found mainly in puffer fish and other marine and terrestrial animals. TTX blocks voltage-gated sodium channels (VGSCs) which are typically classified as TTX-sensitive or TTX-resistant channels. VGSCs play a key role in pain signaling and some TTX-sensitive VGSCs are highly expressed by adult primary sensory neurons. During pathological pain conditions, such as neuropathic pain, upregulation of some TTX-sensitive VGSCs, including the massive re-expression of the embryonic VGSC subtype NaV1.3 in adult primary sensory neurons, contribute to painful hypersensitization. In addition, people with loss-of-function mutations in the VGSC subtype NaV1.7 present congenital insensitive to pain. TTX displays a prominent analgesic effect in several models of neuropathic pain in rodents. According to this promising preclinical evidence, TTX is currently under clinical development for chemo-therapy-induced neuropathic pain and cancer-related pain.
- tetrodotoxin
- TTX
- voltage-gated sodium channels
- neuropathic pain
- cancer pain
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
2. Role of TTX-Sensitive Voltage-Gated Sodium Channels (VGSCs) in Neuropathic and Cancer Pain
The VGSCs are integral membrane proteins composed of a 260 kDa α-subunit and one or more β-subunits, being the α-subunit responsible for forming the pore and for the main biophysical properties of the channel [16]. Most VGSCs are located at peripheral and/or central nervous system (see Figure 1), and are responsible for generating the Na+ currents that lead to the initiation and propagation of neuronal action potentials [17]. TTX binds to VGSCs by the interaction between the positively charged guanidine group in the TTX molecule with the negatively charged carboxylate residues that are placed around the outer vestibule of the channel; this binding occludes the channel pore and blocks Na+ current [18]. VGSCs are classified according to its sensitivity to TTX. Thus, TTX-sensitive VGSCs (NaV1.1, NaV1.2, NaV1.3, NaV1.4, NaV1.6 and NaV1.7) are blocked by nanomolar concentrations of this neurotoxin, whereas TTX-resistant VGSCs (NaV1.5, NaV1.8, NaV1.9) are inhibited at micromolar concentrations [16]. The mechanism of action by which TTX exerts its analgesic effect is by binding to the α-subunit within the outer vestibule of the VGSC, blocking the entry of Na+ ions through the channel [16,18][16][18]. In this manner, TTX reduces the Na+ ionic fluxes required for the initiation and conduction of nerve impulses.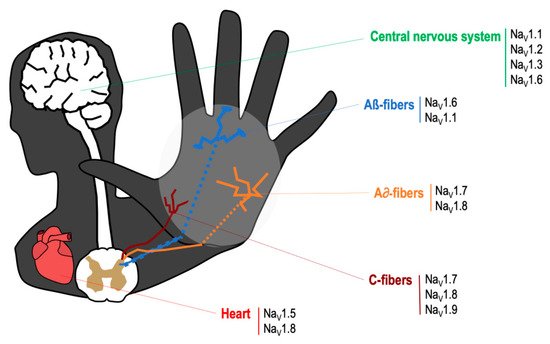
| Channel | Normal Localization [16] | Changes of Expression in Pain States | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | V | 1.1 | -CNS, PNS-Microglia | -Unclear after PNI in NP [16] | |||||
| Na | V | 1.2 | -CNS, very low expression in PNS-SC in lamina I/II | -Unclear after PNI in NP [16] | |||||
| Na | V | 1.3 | -Negligible in DRG (embryonic isoform)-SC in lamina I/II | Upregulated in DRG: CCI [21], PDN [22,23], SNI [24,25], SNL [26,27], traumatic nerve injury (human) [28] Upregulate in painful neuromas (human): [29] Trigeminal ganglion: trigeminal neuropathic pain [30,31,32] Upregulated in sciatic nerve: PDN [33] Upregulated in spinal cord: SCI [34] | Upregulated in DRG: CCI [21], PDN [22][23], SNI [24][25], SNL [26][27], traumatic nerve injury (human) [28] Upregulate in painful neuromas (human): [29] Trigeminal ganglion: trigeminal neuropathic pain [30][31][32] Upregulated in sciatic nerve: PDN [33] Upregulated in spinal cord: SCI [34] |
||||
| Na | V | 1.4 | -Skeletal muscle | ||||||
| Na | V | 1.6 | -Nodes of Ranvier-SC-PNS-Epidermal free nerve terminals-keratinocytes-Microglia | Upregulated in DRG: PDN [35], lumbar 5 ventral root transection [36], CINP (oxaliplatin) [37] Upregulated in sciatic nerve: CCI [38] Upregulated trigeminal ganglion: trigeminal neuropathic pain [30] |
|||||
| Na | V | 1.7 | -PNS in all types of DRG neurons-SC-Epidermal free nerve terminals | Downregulated in DRG: SNL [39], SNI [24,25], traumatic nerve injury (human) [28] Upregulated in DRG: CCI [ Upregulated in spinal cord: CINP (paclitaxel) [42] Upregulated trigeminal ganglion: trigeminal neuropathic pain [44] Upregulated in sciatic nerve: CCI [45] | Downregulated in DRG: SNL [39], SNI [24][25 | 40], SNI [41], SNL [27], CINP (paclitaxel) [42], cancer-related pain (humans) [42], Herpesvirus quiescent infection [43], painful neuromas (human) [ | [27], CINP (paclitaxel) [42], cancer-related pain (humans) [42], Herpesvirus quiescent infection [43 | 34] | ], traumatic nerve injury (human) [28] Upregulated in DRG: CCI [40], SNI [41], SNL ], painful neuromas (human) [34] Upregulated in spinal cord: CINP (paclitaxel) [42] Upregulated trigeminal ganglion: trigeminal neuropathic pain [44] Upregulated in sciatic nerve: CCI [45] |
3. Effects of TTX in Preclinical Models of Neuropathic and Cancer Pain
3.1. Preclinical Studies on Neuropathic Pain
| Administration of TTX | TTX Doses | Effect (None, Moderate, Strong) | Pain Test | Pain Model | Reference |
|---|---|---|---|---|---|
| Sciatic nerve blockade | TTX osmotic pump | Strong | MA, TH | SNI and CCI | [61] |
| Topical DRG | 12.5–50 nM/5 µL | Strong (12.5–50 µg) | MA | SNL | [62] |
| Epidural | 25 nM/5 µL | Strong (25 µg) | MA | SNL | [62] |
| Topical median nerve | Gel pads with TTX | Strong | MA | CCI | [63] |
| Intraperitoneal | 25 nM/5 µL | None | MA | SNL | [62] |
| 8 µg | None | MA | CINP (vincristine) | [64] | |
| Subcutaneous | 1–6 µg | Strong | MA, TH, CA | CINP (paclitaxel) | [65] |
| 6 µg | Strong | MA | intraplantar capsaicin | [66] | |
| 0.3–6 µg | Strong (1–6 µg) | MA, TH | SNL | [57] | |
| Acute and subchronic TTX (1–6 µg) | Strong | MA, TH | CCI | [67] | |
| Acute and subchronic TTX (1–6 µg) | Moderate | MA, TH | CCI-intraorbital nerve | [67] | |
| 8 µg | Strong | MA, TH | burn-induced pain | [68] | |
| Intragastrical | 5–20 µg | Strong | MA, TH | Postherpetic Neuralgia (RTX) | [69] |
| Intramuscular | Acute and subchronic TTX (1–6 µg s.c.) | Strong | MA | Postherpetic Neuralgia (RTX) | [69] |
| 0.03–1 ug | Moderate | MH | CINP (oxaliplatin) | [70] | |
| Intrathecal | 10 µg | Strong | MA, TH | bone cancer pain | [71] |
3.2. Preclinical Studies on Cancer Pain
References
- Sá, K.N.; Moreira, L.; Baptista, A.F.; Yeng, L.T.; Teixeira, M.J.; Galhardoni, R.; de Andrade, D.C. Prevalence of chronic pain in developing countries: Systematic review and meta-analysis. Pain Rep. 2019, 4, e779.
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205.
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers. 2017, 3, 17002.
- Watson, J.C.; Sandroni, P. Central Neuropathic Pain Syndromes. Mayo Clin. Proc. 2016, 91, 372–385.
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32.
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662.
- Van den Beuken-van Everdingen, M.H.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom. Manag. 2016, 51, 1070–1090.
- Caraceni, A.; Shkodra, M. Cancer Pain Assessment and Classification. Cancers 2019, 11, 510.
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173.
- Van den Beuken van Everdingen, M.H.J.; van Kuijk, S.M.J.; Janssen, D.J.A.; Joosten, E.A.J. Treatment of Pain in Cancer: Towards Personalised Medicine. Cancers 2018, 10, 502.
- Haumann, J.; Joosten, E.B.A.; Everdingen, M.H.J.V.D.B. Pain prevalence in cancer patients: Status quo or opportunities for improvement? Curr. Opin. Support Palliat. Care. 2017, 11, 99–104.
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20 (Suppl. 1), S2–S12.
- Cooper, T.E.; Chen, J.; Wiffen, P.J.; Derry, S.; Carr, D.B.; Aldington, D.; Cole, P.; Moore, R.A. Morphine for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 5, CD011669.
- Van den Beuken-van Everdingen, M.H.; de Graeff, A.; Jongen, J.L.; Dijkstra, D.; Mostovaya, I.; Vissers, K.C.; National Guideline Working Group “Diagnosis Treatment of Cancer Pain”. Pharmacological Treatment of Pain in Cancer Patients: The Role of Adjuvant Analgesics, a Systematic Review. Pain Pract. 2017, 17, 409–419.
- Cardoso, F.C.; Lewis, R.J. Sodium channels and pain: From toxins to therapies. Br. J. Pharmacol. 2018, 175, 2138–2157.
- Nieto, F.R.; Cobos, E.J.; Tejada, M.Á.; Sánchez-Fernández, C.; González-Cano, R.; Cendán, C.M. Tetrodotoxin (TTX) as a therapeutic agent for pain. Mar. Drugs 2012, 10, 281–305.
- De Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118.
- Fozzard, H.A.; Lipkind, G.M. The tetrodotoxin binding site is within the outer vestibule of the sodium channel. Mar. Drugs 2010, 8, 219–234.
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926.
- Bennett, D.L.; Clark, A.J.; Huang, J.; Waxman, S.G.; Dib-Hajj, S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019, 99, 1079–1151.
- Li, Y.; Zhang, X.; Fu, Z.; Zhou, Q. MicroRNA-212-3p Attenuates Neuropathic Pain via Targeting Sodium Voltage-gated Channel Alpha Subunit 3 (NaV 1.3). Curr. Neurovasc. Res. 2019, 16, 465–472.
- Cheng, K.I.; Wang, H.C.; Chuang, Y.T.; Chou, C.W.; Tu, H.P.; Yu, Y.C.; Chang, L.L.; Lai, C.S. Persistent mechanical allodynia positively correlates with an increase in activated microglia and increased P-p38 mitogen-activated protein kinase activation in streptozotocin-induced diabetic rats. Eur. J. Pain. 2014, 18, 162–173.
- Hong, S.; Morrow, T.J.; Paulson, P.E.; Isom, L.L.; Wiley, J.W. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. J. Biol. Chem. 2004, 279, 29341–29350.
- Berta, T.; Poirot, O.; Pertin, M.; Ji, R.R.; Kellenberger, S.; Decosterd, I. Transcriptional and functional profiles of voltage-gated Na(+) channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Mol. Cell Neurosci. 2008, 37, 196–208.
- Casals-Díaz, L.; Casas, C.; Navarro, X. Changes of voltage-gated sodium channels in sensory nerve regeneration and neuropathic pain models. Restor. Neurol. Neurosci. 2015, 33, 321–334.
- Black, J.A.; Cummins, T.R.; Plumpton, C.; Chen, Y.H.; Hormuzdiar, W.; Clare, J.J.; Waxman, S.G. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J. Neurophysiol. 1999, 82, 2776–2785.
- Lin, C.R.; Chen, K.H.; Yang, C.H.; Huang, H.W.; Sheen-Chen, S.M. Intrathecal miR-183 delivery suppresses mechanical allodynia in mononeuropathic rats. Eur. J. Neurosci. 2014, 39, 1682–1689.
- Coward, K.; Aitken, A.; Powell, A.; Plumpton, C.; Birch, R.; Tate, S.; Bountra, C.; Anand, P. Plasticity of TTX-sensitive sodium channels PN1 and brain III in injured human nerves. Neuroreport 2001, 12, 495–500.
- Black, J.A.; Nikolajsen, L.; Kroner, K.; Jensen, T.S.; Waxman, S.G. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann. Neurol. 2008, 64, 644–653.
- Liu, M.X.; Zhong, J.; Xia, L.; Dou, N.N.; Li, S.T. IL-6 contributes to Nav1.3 up-regulation in trigeminal nerve following chronic constriction injury. Neurol. Res. 2020, 42, 504–514.
- Liu, M.; Zhong, J.; Xia, L.; Dou, N.; Li, S. The expression of voltage-gated sodium channels in trigeminal nerve following chronic constriction injury in rats. Int. J. Neurosci. 2019, 129, 955–962.
- Yoon, J.H.; Son, J.Y.; Kim, M.J.; Kang, S.H.; Ju, J.S.; Bae, Y.C.; Ahn, D.K. Preemptive application of QX-314 attenuates trigeminal neuropathic mechanical allodynia in rats. Korean J. Physiol. Pharmacol. 2018, 22, 331–341.
- Aghdam, A.M.; Shahabi, P.; Karimi-Sales, E.; Ghiasi, R.; Sadigh-Eteghad, S.; Mahmoudi, J.; Alipour, M.R. Swimming Exercise Induced Reversed Expression of miR-96 and Its Target Gene NaV1.3 in Diabetic Peripheral Neuropathy in Rats. Chin. J. Physiol. 2018, 61, 124–129.
- Liu, J.; Wu, Y. Electro-acupuncture-modulated miR-214 prevents neuronal apoptosis by targeting Bax and inhibits sodium channel Nav1.3 expression in rats after spinal cord injury. Biomed. Pharmacother. 2017, 89, 1125–1135.
- Ren, Y.S.; Qian, N.S.; Tang, Y.; Liao, Y.H.; Yang, Y.L.; Dou, K.F.; Toi, M. Sodium channel Nav1.6 is up-regulated in the dorsal root ganglia in a mouse model of type 2 diabetes. Brain Res. Bull. 2012, 87, 244–249.
- Ding, H.H.; Zhang, S.B.; Lv, Y.Y.; Ma, C.; Liu, M.; Zhang, K.B.; Ruan, X.C.; Wei, J.Y.; Xin, W.J.; Wu, S.L. TNF-α/STAT3 pathway epigenetically upregulates Nav1.6 expression in DRG and contributes to neuropathic pain induced by L5-VRT. J. Neuroinflamm. 2019, 16, 29.
- Li, L.; Shao, J.; Wang, J.; Liu, Y.; Zhang, Y.; Zhang, M.; Zhang, J.; Ren, X.; Su, S.; Li, Y.; et al. MiR-30b-5p attenuates oxaliplatin-induced peripheral neuropathic pain through the voltage-gated sodium channel Nav1.6 in rats. Neuropharmacology 2019, 153, 111–120.
- Tseng, T.J.; Hsieh, Y.L.; Ko, M.H.; Hsieh, S.T. Redistribution of voltage-gated sodium channels after nerve decompression contributes to relieve neuropathic pain in chronic constriction injury. Brain Res. 2014, 1589, 15–25.
- Li, M.; Zhang, S.J.; Yang, L.; Fang, X.L.; Hu, H.F.; Zhao, M.Y.; Li, L.; Guo, Y.Y.; Shao, J.P. Voltage-gated sodium channel 1.7 expression decreases in dorsal root ganglia in a spinal nerve ligation neuropathic pain model. Kaohsiung J. Med. Sci. 2019, 35, 493–500.
- Jia, Q.; Dong, W.; Zhang, L.; Yang, X. Activating Sirt1 by resveratrol suppresses Nav1.7 expression in DRG through miR-182 and alleviates neuropathic pain in rats. Channels 2020, 14, 69–78.
- Tian, J.J.; Tan, C.Y.; Chen, Q.Y.; Zhou, Y.; Qu, Z.W.; Zhang, M.; Ma, K.T.; Shi, W.Y.; Li, L.; Si, J.Q. Upregulation of Nav1.7 by endogenous hydrogen sulfide contributes to maintenance of neuropathic pain. Int. J. Mol. Med. 2020, 46, 782–794.
- Li, Y.; North, R.Y.; Rhines, L.D.; Tatsui, C.E.; Rao, G.; Edwards, D.D.; Cassidy, R.M.; Harrison, D.S.; Johansson, C.A.; Zhang, H.; et al. DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. J. Neurosci. 2018, 38, 1124–1136.
- Zhang, Q.; Martin-Caraballo, M.; Hsia, S.V. Modulation of Voltage-Gated Sodium Channel Activity in Human Dorsal Root Ganglion Neurons by Herpesvirus Quiescent Infection. J. Virol. 2020, 94, e01823-19.
- Yang, K.Y.; Kim, M.J.; Ju, J.S.; Park, S.K.; Lee, C.G.; Kim, S.T.; Bae, Y.C.; Ahn, D.K. Antinociceptive Effects of Botulinum Toxin Type A on Trigeminal Neuropathic Pain. J. Dent. Res. 2016, 95, 1183–1190.
- Stevens, A.M.; Liu, L.; Bertovich, D.; Janjic, J.M.; Pollock, J.A. Differential Expression of Neuroinflammatory mRNAs in the Rat Sciatic Nerve Following Chronic Constriction Injury and Pain-Relieving Nanoemulsion NSAID Delivery to Infiltrating Macrophages. Int. J. Mol. Sci. 2019, 20, 5269.
- Lindia, J.A.; Köhler, M.G.; Martin, W.J.; Abbadie, C. Relationship between sodium channel NaV1.3 expression and neuropathic pain behavior in rats. Pain 2005, 117, 145–153.
- Nassar, M.A.; Baker, M.D.; Levato, A.; Ingram, R.; Mallucci, G.; McMahon, S.B.; Wood, J.N. Nerve injury induces robust allodynia and ectopic discharges in Nav1.3 null mutant mice. Mol. Pain 2006, 2, 33.
- Nassar, M.A.; Levato, A.; Stirling, L.C.; Wood, J.N. Neuropathic pain develops normally in mice lacking both Na(v)1.7 and Na(v)1.8. Mol. Pain 2005, 1, 24.
- Chang, W.; Berta, T.; Kim, Y.H.; Lee, S.; Lee, S.Y.; Ji, R.R. Expression and Role of Voltage-Gated Sodium Channels in Human Dorsal Root Ganglion Neurons with Special Focus on Nav1.7, Species Differences, and Regulation by Paclitaxel. Neurosci. Bull. 2018, 34, 4–12.
- Waxman, S.G.; Merkies, I.S.J.; Gerrits, M.M.; Dib-Hajj, S.D.; Lauria, G.; Cox, J.J.; Wood, J.N.; Woods, C.G.; Drenth, J.P.H.; Faber, C.G. Sodium channel genes in pain-related disorders: Phenotype-genotype associations and recommendations for clinical use. Lancet Neurol. 2014, 13, 1152–1160.
- Xie, W.; Strong, J.A.; Zhang, J.M. Local knockdown of the NaV1.6 sodium channel reduces pain behaviors, sensory neuron excitability, and sympathetic sprouting in rat models of neuropathic pain. Neuroscience 2015, 291, 317–330.
- Chen, L.; Huang, J.; Zhao, P.; Persson, A.K.; Dib-Hajj, F.B.; Cheng, X.; Tan, A.; Waxman, S.G.; Dib-Hajj, S.D. Conditional knockout of NaV1.6 in adult mice ameliorates neuropathic pain. Sci. Rep. 2018, 8, 3845.
- Waxman, S.G.; Dib-Hajj, S.; Cummins, T.R.; Black, J.A. Sodium channels and pain. Proc. Natl. Acad. Sci. USA 1999, 96, 7635–7639.
- Dib-Hajj, S.D.; Fjell, J.; Cummins, T.R.; Zheng, Z.; Fried, K.; LaMotte, R.; Black, J.A.; Waxman, S.G. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain 1999, 83, 591–600.
- Leo, S.; D’Hooge, R.; Meert, T. Exploring the role of nociceptor-specific sodium channels in pain transmission using Nav1.8 and Nav1.9 knockout mice. Behav. Brain Res. 2010, 208, 149–157.
- Zimmer, T.; Haufe, V.; Blechschmidt, S. Voltage-gated sodium channels in the mammalian heart. Glob. Cardiol. Sci. Pract. 2014, 2014, 449–463.
- Marcil, J.; Walczak, J.S.; Guindon, J.; Ngoc, A.H.; Lu, S.; Beaulieu, P. Antinociceptive effects of tetrodotoxin (TTX) in rodents. Br. J. Anaesth. 2006, 96, 761–768.
- Hong, B.; He, J.; Sun, J.; Le, Q.; Bai, K.; Mou, Y.; Zhang, Y.; Chen, W.; Huang, W. Analgesia Effect of Enteric Sustained-Release Tetrodotoxin Pellets in the Rat. Pharmaceutics 2020, 12, 32.
- Alguacil, L.F.; Pérez-García, C.; Salas, E.; González-Martín, C.; Castillo, C.; Polanco, M.J.; Herradón, G.; Morales, L. Subcutaneous tetrodotoxin and inflammatory pain. Br. J. Anaesth. 2008, 100, 729–730.
- González-Cano, R.; Tejada, M.Á.; Artacho-Cordón, A.; Nieto, F.R.; Entrena, J.M.; Wood, J.N.; Cendán, C.M. Effects of Tetrodotoxin in Mouse Models of Visceral Pain. Mar. Drugs 2017, 15, 188.
- Xie, W.; Strong, J.A.; Meij, J.T.A.; Zhang, J.M.; Yu, L. Neuropathic pain: Early spontaneous afferent activity is the trigger. Pain 2005, 116, 243–256.
- Lyu, Y.S.; Park, S.K.; Chung, K.; Chung, J.M. Low dose of tetrodotoxin reduces neuropathic pain behaviors in an animal model. Brain Res. 2000, 871, 98–103.
- Chen, J.J.; Lue, J.H.; Lin, L.H.; Huang, C.T.; Chiang, R.P.; Chen, C.L.; Tsai, Y.J. Effects of pre-emptive drug treatment on astrocyte activation in the cuneate nucleus following rat median nerve injury. Pain 2010, 148, 158–166.
- Nozaki-Taguchi, N.; Chaplan, S.R.; Higuera, E.S.; Ajakwe, R.C.; Yaksh, T.L. Vincristine-induced allodynia in the rat. Pain 2001, 93, 69–76.
- Nieto, F.R.; Entrena, J.M.; Cendán, C.M.; Del Pozo, E.; Vela, J.M.; Baeyens, J.M. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain 2008, 137, 520–531.
- Entrena, J.M.; Cobos, E.J.; Nieto, F.R.; Cendán, C.M.; Gris, G.; Del Pozo, E.; Zamanillo, D.; Baeyens, J.M. Sigma-1 receptors are essential for capsaicin-induced mechanical hypersensitivity: Studies with selective sigma-1 ligands and sigma-1 knockout mice. Pain 2009, 143, 252–261.
- Kayser, V.; Viguier, F.; Ioannidi, M.; Bernard, J.F.; Latrémolière, A.; Michot, B.; Vela, J.M.; Buschmann, H.; Hamon, M.; Bourgoin, S. Differential anti-neuropathic pain effects of tetrodotoxin in sciatic nerve-versus infraorbital nerve-ligated rats—Behavioral, pharmacological and immunohistochemical investigations. Neuropharmacology 2010, 58, 474–487.
- Salas, M.M.; McIntyre, M.K.; Petz, L.N.; Korz, W.; Wong, D.; Clifford, J.L. Tetrodotoxin suppresses thermal hyperalgesia and mechanical allodynia in a rat full thickness thermal injury pain model. Neurosci. Lett. 2015, 607, 108–113.
- Hong, B.; Sun, J.; Zheng, H.; Le, Q.; Wang, C.; Bai, K.; He, J.; He, H.; Dong, Y. Effect of Tetrodotoxin Pellets in a Rat Model of Postherpetic Neuralgia. Mar. Drugs 2018, 16, 195.
- Alvarez, P.; Levine, J.D. Antihyperalgesic effect of tetrodotoxin in rat models of persistent muscle pain. Neuroscience 2015, 311, 499–507.
- Zheng, Q.; Fang, D.; Cai, J.; Wan, Y.; Han, J.S.; Xing, G.G. Enhanced excitability of small dorsal root ganglion neurons in rats with bone cancer pain. Mol. Pain 2018, 8, 24.
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 2019, 160 (Suppl. 1), S1–S10.
- Klein, T.; Magerl, W.; Rolke, R.; Treede, R.D. Human surrogate models of neuropathic pain. Pain 2005, 115, 227–233.
- Bennett, M.I.; Kaasa, S.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D.; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic cancer-related pain. Pain 2019, 160, 38–44.
