Anticancer nanomedicines have been studied over 30 years, but fewer than 10 formulations have been approved for clinical therapy today. Despite abundant options of anticancer drugs, it remains challenging to have agents specifically target cancer cells while reducing collateral toxicity to healthy tissue. Nanocompartments that can be selective toward points deeply within malignant tissues are a promising concept, but the heterogeneity of tumor tissue, inefficiency of cargo loading and releasing, and low uniformity of manufacture required from preclinical to commercialization are major obstacles. Technological advances have been made in this field, creating engineered nanomaterials with improved uniformity, flexibility of cargo loading, diversity of surface modification, and less inducible immune responses.
- nanoparticles
- nanomedicines
- nanomaterials
- nanotechnology
1. Introduction
The National Institutes of Health (NIH) defines nanoparticles as structures ranging from 1 to 100 nm in at least one dimension, while current nanoparticles in therapeutic application are acceptable up to hundreds of nm. Considering the tissue junction between capillaries (150–200 μm), nanoscale structures exhibit unique properties to enhance reactive areas as well as across cell or tissue barriers [1]. For pharmacokinetic properties, the optimal size of nanoparticles is around 100 nm in a hydrodynamic diameter.
Currently, nanoparticles are applied to conventional drugs to improve their efficacy and reduce morbidity for advanced cancer therapies. Antitumor cargos are either capsuled or covalently linked to the nanocarrier. The advantage of covalent links is a precise number of therapeutical molecules for each nanoparticle, while the encapsulation of materials provides more flexibility. Many antitumor drugs are hydrophobic, posing challenges for physiological uptake (Table 1).
| Drug | Solubility (in Water; 25 °C) |
Clinical Use |
|---|---|---|
| Hydrophobic | ||
| Docetaxel | insoluble (<0.3 μg/mL) | Breast, prostate, non-small cell lung cancer, carcinoma, and adenocarcinoma |
| Paclitaxel | insoluble (<0.3 μg/mL) | AIDS-related Kaposi sarcoma, breast, ovarian, and non-small cell lung cancer |
| Alitretinoin | 0.6 μg/mL | Acute promyelocytic leukemia, and AIDS-related Kaposi sarcoma |
| Etoposide | 0.03 mg/mL | Small cell lung and testicular cancer |
| Cisplatin | 2.5 mg/ml | Testicular, ovarian, breast, glioblastoma, non-small cell lung cancer, malignant mesothelioma, and lymphoma |
| Methotrexate | 2.6 mg/mL | ALL, breast, and lung, head and neck cancer, non-Hodgkin lymphoma, and osteosarcoma |
| Fludarabine | 3.53 mg/mL | CLL |
| Doxorubicin | 10 mg/mL | ALL, AML, neuroblastoma, soft tissue and bone sarcomas, breast, ovary, urinary bladder, thyroid, gastric, thyroid, gastric cancer, Hodgkin’s disease |
| Irinotecan HCL | 25 mg/mL | Colon, and rectal cancer |
| Cyclophosphamide | 15.1 mg/mL | ALL, AML, CLL, CML, breast cancer, Hodgkin lymphoma, multiple myeloma, and neuroblastoma |
| Gemcitabine | 51.3 mg/mL | Pancreatic, breast, ovarian, and non-small cell lung cancer |
| Hydrophilic |
Besides stabilizing anticancer agents, designed nanoparticles can also enhance the delivery efficacy by targeting cancer lesions. This concept led to variable nanoparticle designs fitting physicochemical properties via surface modification for a multitude of biomedical applications. The targeting ability of nanoparticles, either passive or active, is aimed for enhancement of drug concentration within the specific tissue of interest, such as tumors, while limiting toxicity to healthy organs. Passive targeting depends on pathophysiological characteristics of tumor vessels, enabling nanomaterials to accumulate in the microenvironment. In tumor tissue, fast angiogenesis with highly disorganized and loosened vessel structure leads to enlarged gap junctions between endothelial cells, resulting in enhanced permeability and retention (EPR) effect [2][6]. The EPR effect allows diffusion of molecules less than 400 nm in diameter, which is suitable for nanoscale complex. The other phenomenon generally observed in tumor tissue is the Warburg effect, a local high metabolic and glycolysis rate result in an acidic environment [3][7]. Designed pH-sensitive biocarrier could be stable at physiological pH = 7.4, but rapidly disassembled and released payload once it reaches an acidic microenvironment. Common design of pH-sensitive nanoparticle is based on polymers with pKa in the range of 6.5–7.2, such as poly(L-histidine) (PHis) and poly(β-amino esters).
Unlike passive targeting, active delivery incorporates other high-affinity molecules to recognize cells directly. Active targeting based on surface receptors on target cells has been widely explored since malignant cells upregulate certain tumor-preferred receptors. For example, transferrin receptor (TfR) and folate receptors (FRs) are physiologically expressed on various normal cells but overexpressed in many cancer types in response to their higher metabolic rate [4][5][10,11].
Conjugation is the process to join the recognition molecules with the therapeutical complex, including direct conjugation or indirect method via linker. One of the main challenges in conjugation design is homogeneity of the molecules. By using a hydrazone ligation, Dawson et al. synthesized viral nanoparticles and conjugated with VEGFR-1 ligand (F56f peptide) on benzaldehyde cowpea mosaic virus nanoparticle for tumor targeting and imaging [6][24]. Moreover, considering orientations of ligands or antibodies; thus, conjugation via linker chemistry is better than direct conjugation for targeting molecules to nanoparticle.
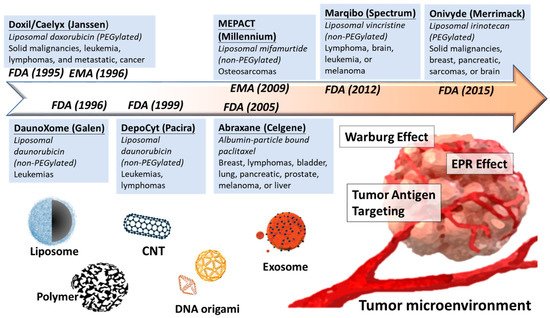
Overall, development of nanomedicine from past decades is a proof of concept to selectively increase the concentration of anticancer agents in tumor malignancy but minimize the side effect from healthy tissues (Figure 1).
2. Current Materials in Nanomedicine
2.1. Lipocomplex
Liposomal nanocomplex is the first delivery tool since the first discovery in the 1960s by A.D Bangham’s group. Liposome formulation ranges from 50 to 200 nm with spherical vesicles composed of phospholipids, and steroids form bilayers in aqueous media can benefit as biocarriers [7][8][35,36]. The properties of liposome were simply applied to increase the solubility of hydrophobic molecules and accelerate physiological metabolism in the beginning. For example, plenty of liposome formulations tried to fit numerous biochemical agents and provide less toxic than the free form. Liposomes were used to deliver lysophosphatidic acids and its analog which regulate normal or malignant blood cell differentiation and proliferation [9][10][37,38]. However, the liposomal formulations in this period faced a severe problem of short pharmacokinetic half-life, until the “stealth liposomes” was designed the 1990s. The second generation of liposome introduced the surface polyethylene glycol (PEG) coating, which highly improved stability and longer circulation time by alleviating the uptake of macrophages [11][12][39,40]. The PEGylation, constructed with a hydrophilic film on surface, can protect the liposome from clearance of reticuloendothelial system, making liposomal delivery clinical practical.
Several lipid complexes have been approved for clinical treatment after fifty years studying of lipocomplex (Table 24).
| Name | Particle Base | Anticancer Drug | Cancer Type | Approval | |
|---|---|---|---|---|---|
| Liposome-based | |||||
| Doxil/Caelyx | (Janssen) | PEGylated liposome | Doxorubicin | Ovarian, breast cancer, leukemia | FDA, 1995 |
| DaunoXome | (Galen) | Non-PEGylated liposome | Daunorubicin | HIV-related Kaposi sarcoma | FDA, 1996 |
| DepoCyt | (Pacira) | Non-PEGylated liposome | Cytarabine | AML, non-Hodgkin lymphoma | FDA,1999 |
| Myocet | (Teva UK) | Non-PEGylated liposome | Doxorubicin | Metastatic breast cancer | EMA, 2000 |
| Marqibo | (Spectrum) | Non-PEGylated liposome | Vincristine | Ph-ALL, Non-Hodgkin’s lymphoma | FDA, 2012 |
| Onivyde | (Merrimack) | PEGylated liposome | irinotecan | Breast, pancreatic, sarcomas, or brain | FDA, 2015 |
| Polymer-based | |||||
| Oncaspar | (Sigma Tau) | PEGylation | L-asparaginase | ALL | FDA,1994 |
| Abraxane | (Celgene) | Albumin-bound polymer | Paclitaxel | Metastatic pancreatic cancer | FDA, 2005 |
2.2. Polymeric and Dendrimer Nanoparticles
Polymeric nanoparticles (PNPs) are structures with a diameter ranging from 10 to 100 nm, which was made from synthetic polymers (e.g., polycaprolactone and polyacrylate) or natural polymers (e.g., albumin, chitosan, and gelatin) [13][14][60,61]. Clinical application of PNPs has reduced ionic surface to avoid the immunological response, while the immobilization of drug within PNPs can increase the drug stability as well. For example, docetaxel-loaded polymeric micelle (diameters < 30 nm) can reach poorly permeable pancreatic tumors in vivo [15][62]. The enhanced stability of the immobilized drug is attributed to the interaction with the polymer carriers to avoid the degradation. Once the complex reaches the target tissues, release mechanism would be triggered by tumor microenvironment. Several unique properties of tumor microenvironment have been used for cargo releasing, such as acidic, hyperthermia, and special enzymes secretion in the local environment. The pH-sensitive polymers are relative stable at a physiologic pH of 7.4 but can be rapidly destructured and can release active drugs in acidic tumor tissues. For example, poly(lactide-co-glycolide) (PLGA) polymer performed as 2–4-fold doxorubicin release in tumor-bearing tissue than circulation at pH 7.4 [16][63]. Moreover, thermosensitive polymeric, such as poly (N-isopropylacrylamide- co-acrylamide-co-allylamine) (PNIPAM-AAm-AA), could be a potential anticancer drug nanocarrier. Under the hyperthermia of tumor region, the hairy structure of PNIPAM-AAm-AA polymer would shrink, while the enclosed doxorubicin releases rapidly [17][18][64,65].
Dendrimer is a unique structure of polymer, which was first synthesized by Vogtle group in 1978, with branched 3D structure that provided a high degree of functional surface [19][68]. This multifunctional property provides the dendrimers more loading space for cargos and interaction with target cells. The cytotoxicity of dendrimer carrier depends on its surface area and the arms of dendrimer, while exchanging the amine groups into hydroxyl group may result in lower levels of cytotoxicity in vivo. The drug could be loaded into the internal structure of dendrimers or covalently linked to dendrimers molecule. Compared to the linear polymers with stochastic structures, dendrimers offer a well-defined size and structure, performing a more precise polyvalence and molecular weight. The polyvalence defines the exact number of active groups on a single dendrimer. By controlling the number of covalent bonds within a single molecule, the quantity of drug loading could be adjusted. Noncovalent encapsulation is an alternative method only when payload is labile or poorly soluble. Poly(amido amide) (PAMAM), a very common dendrimer widely used in biomedical applications, is easily to have molecular conjugation through its branches of amine terminals [20][69]. Thioaptamer (TA)-modified PAMAM is developed to target CD44+ (TA receptor positive) breast cancer in vitro and in vivo by using ligand-receptor affinity [21][70]. Moreover, introducing a folic-acid conjugation has been reported to improve the delivery of PAMAM dendrimers loaded with 2-methoxyestradiol to target KB carcinoma cells overexpressing high-affinity folic acid receptors [22][71].
2.3. Carbon Nanomaterials
Carbon nanotubes (CNT), widely used as nanocarriers, are characterized by the unique structure with the rolling of a single (SWCNTs—single-walled carbon nanotubes) or multi (MWCNTs—multiwalled carbon nanotubes) sheet of graphite with an enormous surface area and an excellent electronic and thermal conductivity [23][76]. The compatibility of nanotube could improve biomedical reagent delivery with advanced chemical modification on its surface. SWCNT has a defined wall, whereas MWCNT mostly has structural defects which result in a less stable nanostructure [24][77]. SWCNTs is a one-dimensional nanomaterial composed of a single graphene layer of cylinder shape in a diameter of 1–2 nm and a length ranging from 50 nm to hundreds of μms. SWCNTs exhibit higher accumulation in tumor tissues physiologically, and their needle-like shape facilitates transmembrane penetration and internalization of therapeutic cargos. Moreover, a high surface area enhances ability to encapsulate and load cargo onto their surface or within their interior core via both covalent and noncovalent linkage. As drug carriers, there remain advantages and disadvantages of SWCNT relative to MWCNT. The stronger structure of SWCNT might be suitable for quality control of delivery, while the low stability of MWCNT makes it easier for further modification. Al Faraj et al. have recently demonstrated enhancement of delivery of doxorubicin by antibody-conjugated magnetic SWCNTs, which can also perform as a noninvasive imaging biomarker [25][26][78,79]. A. Pistone et al. have currently demonstrated hydroxyapatite-magnetite with MWCNT as a biocompatible magnetic drug delivery system in bone tissue engineering [27][80].
