Post-translational modifications play a fundamental role in regulating protein function and stability. In particular, protein ubiquitylation is a multifaceted modification involved in numerous aspects of plant biology. Regarding proteolytic functions of Ub, Lys-48-linked branched chains are the most common chain type for proteasomal degradation, whereas promotion of endocytosis and vacuolar degradation is triggered through monoubiquitylation or Lys63-linked chains introduced in integral or peripheral plasma membrane proteins. Hormone signaling relies on regulated protein turnover, and specifically the half-life of ABA signaling components is regulated both through the ubiquitin-26S proteasome system and the endocytic/vacuolar degradation pathway.
- abscisic acid
- ABA receptor
- clade A PP2C
- E3 ubiquitin ligases
- PYR/PYL/RCAR
- RBR E3 ligase
- CRL3
- CRL4
- RING E3 ligase
- PUB E3 ligase
1. Introduction
The Ub-26S proteasome (UPS) and vacuolar degradation pathways play fundamental roles in the regulation of protein half-life and control of protein homeostasis in plant cells [1][2][3][1,2,3]. The UPS first catalyzes the attachment of Ub molecules to selected targets, and this promotes their degradation by the multiprotease 26S proteasome complex [2]. The E1 (Ub-activating enzyme, UBA), E2 (Ub-conjugating enzyme, UBC), and E3 (Ub ligase) cascade is involved in conjugating Ub to substrate proteins [2][4][2,7]. The Arabidopsis genome contains 2 UBAs, 37 UBCs, and more than 1400 E3 ligases, which are divided in monomeric (HECT, RING, RBR, U-box type E3s) and multimeric E3 ligases [4][7]. These latter E3s are assembled using Cul1, Cul3a/3b, or Cul4 scaffold proteins, which bind the RING box-1 (RBX1) protein for recruitment of the E2 UBC in the cullin (CUL)-RING E3 ubiquitin ligase (CRL) complex. Additionally, multimeric CRLs require substrate adaptors for recognition of the target proteins. Cul1-based E3 complexes are usually referred to as SCF (for SKP1-Cullin-F box), whereas Cul3- and Cul4-based complexes are known as CRL3 and CRL4, respectively. On the other hand, the endosomal trafficking pathway plays a key role in the turnover of membrane proteins and requires components of the endosomal sorting complex required for transport (ESCRT) machinery, such as the FYVE domain-containing protein 1 (FYVE1)/FYVE domain protein required for endosomal sorting 1 (FREE1) and vacuolar protein sorting 23A (VPS23A) members of the ESCRT-I complex, and ALG-2 interacting protein-X (ALIX) associated protein of ESCRT-III.
2. Ubiquitylation of ABA Receptors
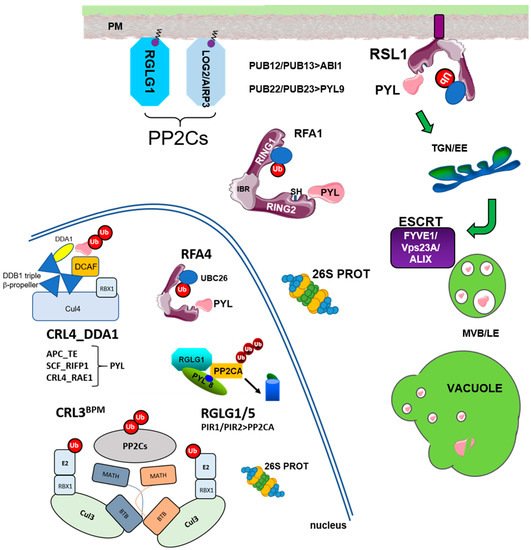
Ubiquitylation of ABA receptors was reported by Bueso et al. and Irigoyen et al. [5][6][8,9] using affinity purification of ubiquitinated proteins with p62-agarose and immuno-analysis of protein extracts prepared from MG132-treated plants, which expressed HA-tagged versions of PYR1, PYL4, and PYL8. Additionally, these authors identified E3 ubiquitin ligases that target ABA receptors in the plasma membrane (PM), namely RSL1 for PYR1 and PYL4 [5][8], or in the nucleus, through the multimeric CRL4 E3 ligase that uses DET1 and DDB1-ASSOCIATED1 (DDA1) as part of a substrate adaptor module for recognition of PYL8 and PYL9 [6][9]. The RSL1- and CRL4DDA1-dependent ubiquitination pathways point to two different mechanisms for receptor degradation: endosome-mediated vacuolar degradation and 26S proteasome, respectively.
RSL1 is anchored tomembrane through a C-terminal transmembrane domain (TMD) and originally was annotated as a RING-type E3 ligase [5][8]; however, further inspection of the RSL1 protein reveals three putative RING domains in tandem, named as RING1-IN BETWEEN RING (IBR)-RING2, and accordingly, RSL1 belongs to the RBR-type E3 ligase family [7][8][9][10,11,12]. Ubiquitylation in the PM triggers endocytosis of integral membrane proteins (nutrient transporters, ion channels, signaling receptors) or proteins associated to the PM [10][11][12][13,14,15]. The turnover of ABA receptors in the proximity of the PM likely plays an important role in regulating ABA effects on different ion and water transporters [13][14][15][16,17,18]. Upon endocytosis, ABA receptors follow sorting through the ESCRT machinery and finally vacuolar degradation [5][16][17][18][8,19,20,21]. At least three components of the ESCRT machinery, namely VPS23, FYVE1/FREE, and ALIX (Figure 1), have been involved in the transit of ubiquitinated ABA receptors through the ESCRT machinery. Knock-down mutations in these ESCRT components lead to attenuated degradation of ABA receptors and enhanced ABA signaling [16][17][18][19,20,21].
3. Regulation of Ubiquitylation by Post-Translational Modifications
Mass spectrometry analysis of ABA receptors and ABA-activated SnRK2s has identified different post-translational modifications [24][25][26][36,37,38]. Such modifications seem to regulate protein activity in a dual manner, namely by affecting biochemical function of the signaling proteins and also by promoting ubiquitylation and protein degradation. For example, ABA induces phosphorylation and activation of SnRK2s by B2/B3-type RAF kinases, but as a side effect, degradation of SnRK2s in promoted by ABA [25][37]. Interestingly, when phosphorylation of SnRK2s is abolished in the high-order RAF mutants (namely OK100-oct and OK100-nonu mutants), ABA-induced SnRK2 degradation is prevented [25][37]. For ABA receptors, phosphorylation usually downregulates their activity and additionally promotes their degradation [26][27][28][38,39,40]. For example, phosphorylation of ABA receptors by Arabidopsis EL1-like (AEL) casein kinases leads to their destabilization and the suppression of the ABA response [27][39]. CEPR2 also phosphorylates and accelerates the degradation of ABA receptors [28][40]; thus, the receptor-like kinase CEPR2, rather than participating in a phosphorylation cascade for signaling, exerts direct control of receptor activity and stability.
4. Ubiquitylation of Clade A PP2Cs in the Context of ABA Signaling
ABA receptors play a double inhibitory role on clade A PP2Cs because, in addition to inhibiting PP2C activity, the degradation of certain PP2Cs promoted by E3 Ub ligases is also facilitated by the formation of ternary complexes with ABA and ABA receptors [19][29][25,42]. The regulation of protein activity and stability of clade A PP2Cs is crucial in ABA signaling because these proteins are key repressors of the pathway [30][43]. Thus, the ubiquitylation of clade A PP2Cs enhanced by ABA, and ABA receptors help in the activation of the ABA response. According to recent discoveries in the ABA pathway, we can distinguish several steps in ABA signaling: (i) PP2C inhibition by ABA receptors in an ABA-dependent manner, (ii) activation of subfamily III SnRK2.2/3/6 kinases (SnRK2s) by phosphorylation of B2 and B3 RAFs at Ser171 and Ser175 residues (SnRK2.6/OST1 nomenclature), (iii) amplification of SnRK2 activation by intermolecular trans-phosphorylation of SnRK2s by already active SnRK2s, (iv) ABA response in the PM and nucleus through SnRK2-dependent phosphorylation of different targets and lack of PP2C inhibition on them, including ion transporters and ABRE-binding factors (ABFs), (v) ABF-mediated induction of clade A PP2Cs as a negative feedback mechanism that involves the accumulation of newly synthesized PP2Cs, and (vi) finally the resetting to basal PP2C protein levels (PP2C proteostasis).
5. ABA- and/or Receptor-Dependent Degradation of PP2Cs
The pioneering work of Kong et al. [29][42] demonstrated that clade A PP2C ABI1 is degraded by the 26S proteasome. To determine which E3 ligases target ABI1 for degradation, the authors tested as putative preys in Y2H assays several candidates reported to interact with ABI5 (such as DWA1/2 or KEG), others involved in stress response (such as RGLG1/2 and SDIR1), and 23 plant U-box (PUB) E3 ligases. The authors found that PUB12 and PUB13 interact with ABI1 using different assays, and peptides of PUB12 and PUB13 could be recovered in coIP/MS proteomic analyses of immunoprecipitated ABI1. PUB12 and 13 promote the ubiquitylation of ABI1 in a receptor-dependent manner, which is strictly dependent on exogenous ABA for the dimeric PYR1 receptor or enhanced for the monomeric PYL4/PYL9 receptors [29][42]. Using specific antibodies against ABI1, the authors found that the ABI1 protein level was higher in the PUB12 PUB13 double mutant than in WT, and that ABA enhanced the degradation of ABI1. PUB12 and 13 were previously reported to ubiquitinate the receptor-like kinase FLS2 located in the PM, which mediates flagellin-induced FLS2 endocytosis/degradation [31][46]. Flagellin induces recruitment of PUB12 and PUB13 to the FLS2 receptor complex through BAK1-mediated phosphorylation of the E3 ligases. By analogy, it is tempting to speculate that ABI1 might be ubiquitinated by PUB12/13 in the proximity of the PM when receptor-ABA-ABI1 complexes are formed.
Different E3 ligases can target the same substrate depending on the physiological and cellular context, and the integration of distinctive types of E3 ligases has been reported to act simultaneously on the same client substrates [32][33][49,50].
Interestingly, BPMs are nuclear proteins that function as substrate adaptors of the multimeric CRL3 E3 ligases. In particular, CRL3BPM complexes play a fundamental role in the stress response and hormone signaling through the regulation of different nuclear targets [20][34][35][26,52,53]. PP2CA accumulates predominantly in the nucleus, although interaction of PP2CA with ion transporters of the PM has also been reported [15][36][18,54].
Recently, the previously described LOSS OF GDU2 (LOG2)/ABA-INSENSITIVE RING PROTEIN3 (AIRP3) E3 ligase has also been found to be involved in the regulation of ABI1 levels [37][38][39][55,56,57]. This RING-type E3 ligase is myristoylated, and its membrane localization is important for the interaction with the amino acid exporter regulatory subunit GLUTAMINE DUMPER1 (GDU1) [39][57]. However, the results of Pratelli et al. [39][57] do not support that ubiquitination of GDU1 by LOG2 leads to GDU1 degradation; instead, their data suggest that LOG2 activates GDU1 via ubiquitination, and subsequently, the activated GDU1 facilitates amino acid efflux. On the other hand, the AIRP3/LOG2 transcript is upregulated by drought and ABA, and the AIRP3 mutant is impaired in the abiotic stress response [37][55]. A reasonable explanation for this phenotype could be the lack of ABI1 degradation, as AIRP3 (in concert with UBC27) was recently reported to interact with ABI1 and promote its degradation [38][56]. The subcellular localization of the AIRP3–ABI1 interaction was not investigated [38][56]; therefore, it is intriguing to unveil whether AIRP3 follows a similar mechanism to myristoylated RGLG1 and might interact with ABI1 in the PM (as the LOG2-GDU1 interaction) or in the nucleus upon translocation in response to ABA.
