Pyrazolo[1,5-a]pyrimidine (PP) derivatives are an enormous family of N-heterocyclic compounds that possess a high impact in medicinal chemistry and have attracted a great deal of attention in material science recently due to their significant photophysical properties.
- antitumor scaffold
- enzymatic inhibitory
- N-heterocyclic compounds
- organic synthesis
- pyrazolo[1
- 5-a]pyrimidine
- functionalization
1. Introduction
Pyrazolo[1,5-a]pyrimidine (PP) structural motif is a fused, rigid, and planar N-heterocyclic system that contains both pyrazole and pyrimidine rings [1]. This fused pyrazole is a privileged scaffold for combinatorial library design and drug discovery because its great synthetic versatility permits structural modifications throughout its periphery. The PP derivatives synthesis has been widely studied; thus, various reviews related to the obtention and later derivatization steps have been described in the literature [2,3[2][3][4][5],4,5], after the first critical review involving this attractive scaffold [6] (Figure 1).
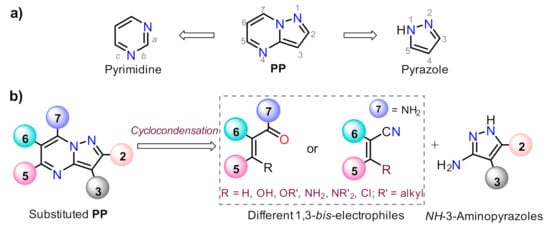
Figure 1. Pyrazolo[1,5-a]pyrimidine with (a) the constituent rings and (b) the modified periphery in accordance with the retrosynthetic analysis.
Despite those reports, the synthetic transformations involving this motif still represent a research priority regarding the process efficiency, environmental impact, and the study of its multiple applications. These reports should address protocols that aim to minimize synthesis pathways, employ cheap reagents and develop processes that prevent or reduce waste production. Usually, PP derivative synthesis involves the pyrimidine ring construction via the interaction of NH-3-aminopyrazoles with different 1,3-biselectrophilic compounds such as β-dicarbonyls, β-enaminones, β-haloenones, β-ketonitriles, and so on (Figure 1b) [1,2,3,4,5,6][1][2][3][4][5][6]. Pyrazolo[1,5-a]pyrimidine scaffold is part of bioactive compounds with exceptional properties like selective protein inhibitor [7], anticancer [8], psychopharmacological [9], among others [10,11][10][11]. Furthermore, the biocompatibility and lower toxicity levels of PP derivatives have led them to reach commercial molecules, for instance, Indiplon, Lorediplon, Zaleplon, Dorsomorphin, Reversan, Pyrazophos, Presatovir, and Anagliptin (Figure 2) [1,2,3,4,5][1][2][3][4][5]. In recent years, this molecular motif has been a focus of study for promising new applications related to materials sciences [12[12][13][14][15][16][17][18][19][20][21],13,14,15,16,17,18,19,20,21], due to its exceptional photophysical properties as an emergent fluorophore [15,16,17,18,19,20,21][15][16][17][18][19][20][21]. Likewise, the tendency of pyrazolo[1,5-a]pyrimidine derivatives to form crystals with notable conformational and supramolecular phenomena [17,22,23][17][22][23] could amplify their applications towards the solid-state. Therefore, we aim to cover two main topics related to compounds bearing the pyrazolo[1,5-a]pyrimidine core. At the first one, the reader will find relevant synthesis strategies and functionalization reactions. Subsequently, in the second part, we focus on the recent compounds presenting antitumoral and enzymatic inhibitory activity. The examples described and commented herein came from the 2015 to 2021 period.
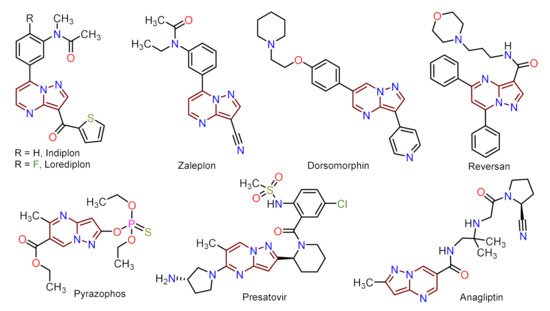
Figure 2.
Molecular structures of commercial compounds bearing the PP motif highlighted in brownish-red.
2. Synthesis and Functionalization
| Synthetic Methods | Substituted PP | Functionalization Reactions | ||
|---|---|---|---|---|
| Section 2.1.1 | 1,3-Biselectrophillic systems: β-dicarbonyls, etc.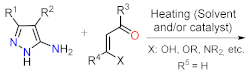 |
 |
Metal catalyzed reactions: Suzuki, etc.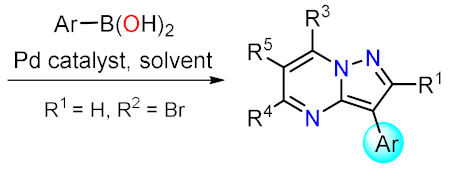 |
Section 2.2.1 |
| Section 2.1.2 | Multicomponent reactions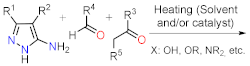 |
Nucleophilic aromatic substitution.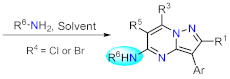 |
Section 2.2.2 | |
| Section 2.1.3 | Synthesis by a pericyclic reaction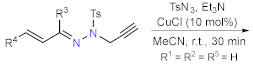 |
Other functionalization reactions. |
Section 2.2.3 | |
| [a] General examples of some relevant synthetic transformations (synthesis and functionalizations) are showed. | ||||
2.1. Synthesis
As stated before, the synthesis of PP derivatives is the focus of various research; however, more recent studies in this area are focused on improving known reaction protocols. Regardless, innovative synthesis methods still emerge that offer creative ways to modify established ones. Notably, the main synthesis route of pyrazolo[1,5-a]pyrimidines allows versatile structural modifications at positions 2, 3, 5, 6, and 7 via the cyclocondensation reaction of 1,3-biselectrophilic compounds with NH-3-aminopyrazoles as 1,3-bisnucleophilic systems (Figure 1b) [1,2,3,4,5,6,24,25][1][2][3][4][5][6][24][25].
2.2. Functionalization
2.2.1. Metal Catalyzed Reactions
Suzuki Couplings
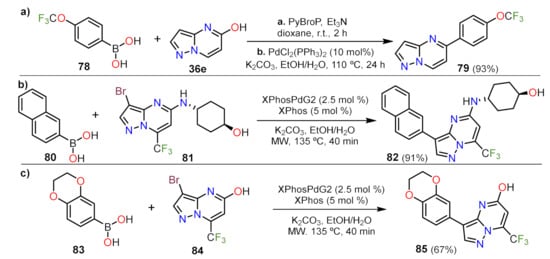
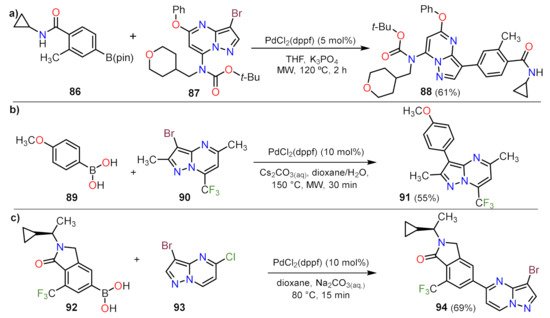
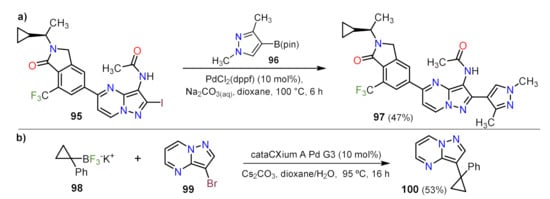
Sonogashira Couplings
Other Metal-Catalyzed Reactions
References
- Castillo, J.C.; Portilla, J. Recent advances in the synthesis of new pyrazole derivatives. Targets Heterocycl. Syst. 2018, 22, 194–223.
- Salem, M.A.; Helal, M.H.; Gouda, M.A.; Abd EL-Gawad, H.H.; Shehab, M.A.M.; El-Khalafawy, A. Recent synthetic methodologies for pyrazolo[1,5-a]pyrimidine. Synth. Commun. 2019, 49, 1750–1776.
- Al-Azmi, A. Pyrazolo[1,5-a]pyrimidines: A Close Look into their Synthesis and Applications. Curr. Org. Chem. 2019, 23, 721–743.
- Cherukupalli, S.; Karpoormath, R.; Chandrasekaran, B.; Hampannavar, G.A.; Thapliyal, N.; Palakollu, V.N. An insight on synthetic and medicinal aspects of pyrazolo[1,5-a]pyrimidine scaffold. Eur. J. Med. Chem. 2017, 126, 298–352.
- Eftekhari-Sis, B.; Zirak, M. Chemistry of α-oxoesters: A powerful tool for the synthesis of heterocycles. Chem. Rev. 2015, 115, 151–264.
- Abu Elmaati, T.M.; El-Taweel, F.M. New Trends in the Chemistry of 5-Aminopyrazoles. J. Heterocycl. Chem. 2004, 41, 109–134.
- Asano, T.; Yamazaki, H.; Kasahara, C.; Kubota, H.; Kontani, T.; Harayama, Y.; Ohno, K.; Mizuhara, H.; Yokomoto, M.; Misumi, K.; et al. Identification, Synthesis, and Biological Evaluation of 6-[(6R)-2-(4-Fluorophenyl)-6-(hydroxymethyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidin-3-yl]-2-(2-methylphenyl)pyridazin-3(2H)-one (AS1940477), a Potent p38 MAP Kinase Inhibitor. J. Med. Chem. 2012, 55, 7772–7785.
- Zhao, M.; Ren, H.; Chang, J.; Zhang, D.; Yang, Y.; He, Y.; Qi, C.; Zhang, H. Design and synthesis of novel pyrazolo[1,5-a]pyrimidine derivatives bearing nitrogen mustard moiety and evaluation of their antitumor activity in vitro and in vivo. Eur. J. Med. Chem. 2016, 119, 183–196.
- Ramsey, S.J.; Attkins, N.J.; Fish, R.; van der Graaf, P.H. Quantitative pharmacological analysis of antagonist binding kinetics at CRF1 receptors in vitro and in vivo. Br. J. Pharmacol. 2011, 164, 992–1007.
- Babaoglu, K.; Boojamra, C.G.; Eisenberg, E.J.; Hui, H.C.; Mackman, R.L.; Parrish, J.P.; Sangi, M.; Saunders, O.L.; Siegel, D.; Sperandio, D.; et al. Pyrazolo[1,5-a]pyrimidines as antiviral agents. Patent WO2011163518A1, 29 December 2011.
- Naidu, B.N.; Patel, M.; D’andrea, S.; Zheng, Z.B.; Connolly, T.P.; Langley, D.R.; Peese, K.; Wang, Z.; Walker, M.A.; Kadow, J.F. Inhibitors of Human Immunodeficiency Virus Replication. Patent WO2014028384A1, 20 February 2014.
- Yang, X.Z.; Sun, R.; Guo, X.; Wei, X.R.; Gao, J.; Xu, Y.J.; Ge, J.F. The application of bioactive pyrazolopyrimidine unit for the construction of fluorescent biomarkers. Dye. Pigment. 2020, 173, 107878.
- Singsardar, M.; Sarkar, R.; Majhi, K.; Sinha, S.; Hajra, A. Brønsted Acidic Ionic Liquid-Catalyzed Regioselective Synthesis of Pyrazolopyrimidines and Their Photophysical Properties. ChemistrySelect 2018, 3, 1404–1410.
- Golubev, P.; Karpova, E.A.; Pankova, A.S.; Sorokina, M.; Kuznetsov, M.A. Regioselective Synthesis of 7-(Trimethylsilylethynyl)pyrazolo[1,5-a]pyrimidines via Reaction of Pyrazolamines with Enynones. J. Org. Chem. 2016, 81, 11268–11275.
- Tigreros, A.; Portilla, J. Fluorescent Pyrazole Derivatives: An Attractive Scaffold for Biological Imaging Applications. Curr. Chinese Sci. 2021, 1, 197–206.
- Tigreros, A.; Portilla, J. Recent progress in chemosensors based on pyrazole derivatives. RSC Adv. 2020, 10, 19693–19712.
- Tigreros, A.; Macías, M.; Portilla, J. Photophysical and crystallographic study of three integrated pyrazolo[1,5-a]pyrimidine–triphenylamine systems. Dye. Pigment. 2021, 184, 108730.
- Tigreros, A.; Aranzazu, S.L.; Bravo, N.F.; Zapata-Rivera, J.; Portilla, J. Pyrazolo[1,5-a]pyrimidines-based fluorophores: A comprehensive theoretical-experimental study. RSC Adv. 2020, 10, 39542–39552.
- Tigreros, A.; Castillo, J.C.; Portilla, J. Cyanide chemosensors based on 3-dicyanovinylpyrazolo[1,5-a]pyrimidines: Effects of peripheral 4-anisyl group substitution on the photophysical properties. Talanta 2020, 215, 120905.
- Tigreros, A.; Rosero, H.A.; Castillo, J.C.; Portilla, J. Integrated pyrazolo[1,5-a]pyrimidine–hemicyanine system as a colorimetric and fluorometric chemosensor for cyanide recognition in water. Talanta 2019, 196, 395–401.
- Castillo, J.C.; Tigreros, A.; Portilla, J. 3-Formylpyrazolo[1,5-a]pyrimidines as Key Intermediates for the Preparation of Functional Fluorophores. J. Org. Chem. 2018, 83, 10887–10897.
- Portilla, J.; Quiroga, J.; Cobo, J.; Low, J.N.; Glidewell, C. Hydrogen-bonded chains in isostruo tural 5-methyl-2-(4-methylphenyl)-7,8-dihydro-6H-cyclopenta[g]pyrazolo[1,5-a]pyrimidine, 2-(4-chloro-phenyl)-5-methyl-7,8-dihydro-6H-cyclopenta[g]pyrazolo[1,5-a]pyrimidine and 2-(4-bromophenyl)-5-methyl-7,8-dihydro-6H-. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2005, 61, 452–456.
- Portilla, J.; Quiroga, J.; Cobo, J.; Low, J.N.; Glidewell, C. 7-Amino-2,5-dimethylpyrazolo[1,5-a]pyrimidine hemihydrate redetermined at 120 K: A three-dimensional hydrogen-bonded framework. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2006, 62, 186–189.
- Regan, A.C. 11.12- Bicyclic 5-6 Systems with One Bridgehead (Ring Junction) Nitrogen Atom: Two Extra Heteroatoms 1:1. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; pp. 551–587. ISBN 978-0-08-044992-0.
- Candeias, N.R.; Branco, L.C.; Gois, P.M.P.; Afonso, C.A.M.; Trindade, A.F. More sustainable approaches for the synthesis of n-based heterocycles. Chem. Rev. 2009, 109, 2703–2802.
- Liu, Y.; Laufer, R.; Patel, N.K.; Ng, G.; Sampson, P.B.; Li, S.-W.; Lang, Y.; Feher, M.; Brokx, R.; Beletskaya, I.; et al. Discovery of Pyrazolo[1,5-a]pyrimidine TTK Inhibitors: CFI-402257 is a Potent, Selective, Bioavailable Anticancer Agent. ACS Med. Chem. Lett. 2016, 7, 671–675.
- Drew, S.L.; Thomas-Tran, R.; Beatty, J.W.; Fournier, J.; Lawson, K.V.; Miles, D.H.; Mata, G.; Sharif, E.U.; Yan, X.; Mailyan, A.K.; et al. Discovery of Potent and Selective PI3KγInhibitors. J. Med. Chem. 2020, 63, 11235–11257.
- Harris, M.R.; Wisniewska, H.M.; Jiao, W.; Wang, X.; Bradow, J.N. A Modular Approach to the Synthesis of gem-Disubstituted Cyclopropanes. Org. Lett. 2018, 20, 2867–2871.
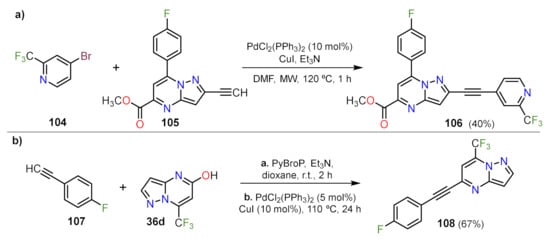
- Jismy, B.; Guillaumet, G.; Allouchi, H.; Akssira, M.; Abarbri, M. Concise and Efficient Access to 5,7-Disubstituted Pyrazolo[1,5-a]pyrimidines by Pd-Catalyzed Sequential Arylation, Alkynylation and SNAr Reaction. Eur. J. Org. Chem. 2017, 2017, 6168–6178.
- Jismy, B.; Tikad, A.; Akssira, M.; Guillaumet, G. Efficient Access to 3,5-Disubstituted 7-(Trifluoromethyl)pyrazolo[1,5-a]pyrimidines Involving SNAr and Suzuki Cross-Coupling Reactions. Molecules 2020, 25, 2062.
- Jismy, B.; Guillaumet, G.; Akssira, M.; Tikad, A.; Abarbri, M. Efficient microwave-assisted Suzuki-Miyaura cross-coupling reaction of 3-bromo pyrazolo[1,5-a] pyrimidin-5(4 H)-one: Towards a new access to 3,5-diarylated 7-(trifluoromethyl)pyrazolo[1,5-a] pyrimidine derivatives. RSC Adv. 2021, 11, 1287–1302.
- Abe, M.; Seto, M.; Gogliotti, R.G.; Loch, M.T.; Bollinger, K.A.; Chang, S.; Engelberg, E.M.; Luscombe, V.B.; Harp, J.M.; Bubser, M.; et al. Discovery of VU6005649, a CNS Penetrant mGlu7/8 Receptor PAM Derived from a Series of Pyrazolo[1,5-a]pyrimidines. ACS Med. Chem. Lett. 2017, 8, 1110–1115.
- Castillo, J.C.; Rosero, H.A.; Portilla, J. Simple access toward 3-halo- and 3-nitro-pyrazolo[1,5-a] pyrimidines through a one-pot sequence. RSC Adv. 2017, 7, 28483–28488.
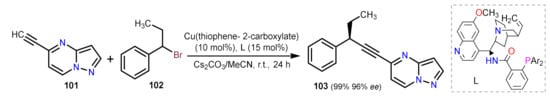
- Dong, X.Y.; Zhang, Y.F.; Ma, C.L.; Gu, Q.S.; Wang, F.L.; Li, Z.L.; Jiang, S.P.; Liu, X.Y. A general asymmetric copper-catalysed Sonogashira C(sp 3)–C(sp) coupling. Nat. Chem. 2019, 11, 1158–1166.
- Childress, E.S.; Wieting, J.M.; Felts, A.S.; Breiner, M.M.; Long, M.F.; Luscombe, V.B.; Rodriguez, A.L.; Cho, H.P.; Blobaum, A.L.; Niswender, C.M.; et al. Discovery of Novel Central Nervous System Penetrant Metabotropic Glutamate Receptor Subtype 2 (mGlu 2 ) Negative Allosteric Modulators (NAMs) Based on Functionalized Pyrazolo[1,5-a]pyrimidine-5-carboxamide and Thieno[3,2-b]pyridine-5-carboxamide Cores. J. Med. Chem. 2019, 62, 378–384.
- Jismy, B.; Allouchi, H.; Guillaumet, G.; Akssira, M.; Abarbri, M. An Efficient Synthesis of New 7-Trifluoromethyl-2,5-disubstituted Pyrazolo[1,5-a]pyrimidines. Synth. 2018, 50, 1675–1686.
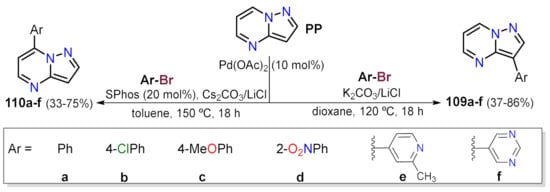
- Bedford, R.B.; Durrant, S.J.; Montgomery, M. Catalyst-Switchable Regiocontrol in the Direct Arylation of Remote C-H Groups in Pyrazolo[1,5-a]pyrimidines. Angew. Chemie Int. Ed. 2015, 54, 8787–8790.
- Loubidi, M.; Manga, C.; Tber, Z.; Bassoude, I.; Essassi, E.M.; Berteina-Raboin, S. One-Pot SNAr/Direct Pd-Catalyzed CH Arylation Functionalization of Pyrazolo[1,5-a]pyrimidine at the C3 and C7 Positions. Eur. J. Org. Chem. 2018, 2018, 3936–3942.
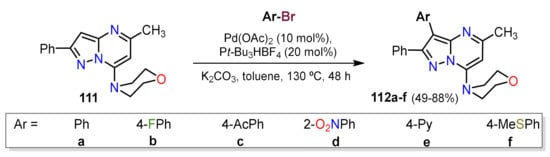
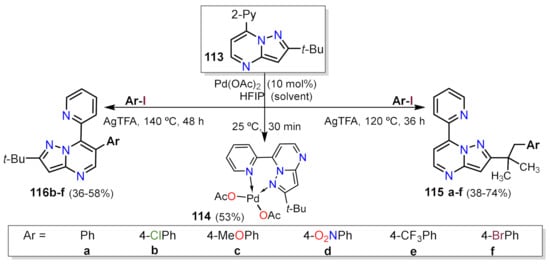
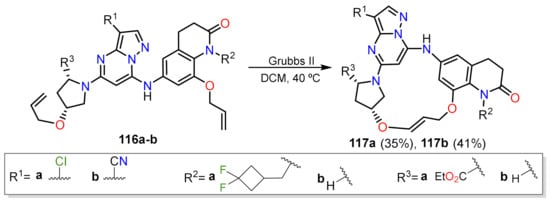
- Gogula, T.; Zhang, J.; Lonka, M.R.; Zhang, S.; Zou, H. Temperature-modulated selective C(sp3)-H or C(sp2)-H arylation through palladium catalysis. Chem. Sci. 2020, 11, 11461–11467.
- Colomer, I.; Chamberlain, A.E.R.; Haughey, M.B.; Donohoe, T.J. Fc12 Hfip. Nat. Rev. Chem. 2017, 1, 0088.
- McCoull, W.; Abrams, R.D.; Anderson, E.; Blades, K.; Barton, P.; Box, M.; Burgess, J.; Byth, K.; Cao, Q.; Chuaqui, C.; et al. Discovery of Pyrazolo[1,5-a]pyrimidine B-Cell Lymphoma 6 (BCL6) Binders and Optimization to High Affinity Macrocyclic Inhibitors. J. Med. Chem. 2017, 60, 4386–4402.
