Irinotecan has been used in the treatment of various malignancies for many years. Still, the knowledge regarding this drug is expanding. The pharmacogenetics of the drug is the crucial component of response to irinotecan. Furthermore, new formulations of the drug are introduced for it enhanced delivery. Novel formulations of drugs (e.g., liposomal formulations, dendrimers, and nanoparticles) have a huge potential to avoid potentially life-threatening side effects of the iriniotecan.
- Irinotecan
- topoisomerase
- cancer
1. Definition
Irinotecan has been used in the treatment of various malignancies for many years. Still, the knowledge regarding this drug is expanding. The pharmacogenetics of the drug is the crucial component of response to irinotecan. Furthermore, new formulations of the drug are introduced in order to better deliver the drug and avoid potentially life-threatening side effects.
2. Introduction
Despite significant progress in medicine, classical chemotherapy still remains the first-line treatment of cancer, especially metastatic tumors. Tumor drug resistance and potential side effects are the main limiting factors in cancer treatment. These factors promote continuous drug development and research that studies the effects of combined treatments of existing drugs. Moreover, the field of drug delivery is expanding rapidly, raising hope for more efficient anticancer therapies [1]. These include the use of liposomal formulations, dendrimers, and nanoparticles as delivery systems. Among conventional antineoplastic drugs, several classes are distinguishable: alkylating agents, antimetabolites, topoisomerase inhibitors, mitotic spindle inhibitors, and others [2][3]. Topoisomerase I inhibitors have been extensively studied since the late 1960s; however, initially, their clinical use was hindered due to their severe toxicity and low stability. Irinotecan as the first member of this drug group was approved for the treatment of cervical, lung, and ovarian cancer in Japan in 1994. In the following years, its use was approved in Europe (1995) and the USA (1996) [4].
3. Irinotecan (CPT-11)
One of the best-studied topoisomerase I inhibitoris is irinotecan han. Since its introduction in 1996, 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothecine (CPT-11) has been used in theto treatment of various mal various types of cancer such as: pulmonary [5][6][7], pancreatic, gnastric [8][9], ovarian [10][11], cerviescal [12], fcolorectal m[13], anyd yearsothers [14]. SIsolated from tillhe Chinese tree Camptotheca acuminata, campthe knowledge regarding this drug is expanding. The phothecin alkaloid was the base for semi-synthetic, water-soluble anologs such as irinotecan (CPT-11, drug name: Camptosar®, Campto®) acnd topogeneticstecan (Hycamtin®) that have been approf the drug is the crucial cved by the US Federal Drug Administration for clinical use (in 1996 and 2007 respectively) [15]. Irinomtecan is a ponent of response to irinotecanentacyclic alkaloid provided with a bis-piperidine side chain, which contributes to its water solubility (Figure 1) [16]. FuSimilarthermore, new formulations to other campthothecins, irinotecan undergoes structural changes depending on the physiological pH of the drug are introduced in ordercellular environment. Irinotecan is considered to better deliver the drug and avo active in lactone structural form but impermeable through cell membranes and therefore inactive in carboxylate form [17][18].
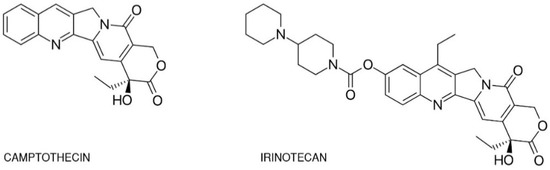
Figure 1. Chemidcal structures of potenticamptothecin and irinotecan.
4. New Irinotecan Formulations
Mallny life-threatening side effectsdifferent approaches were taken to overcome the problem of bioavailability of the drug or its active metabolite SN-38.
D Thespe ite significant progress in medicine, clasnclude the design of liposomal formulations, nanoparticles, polymer conjugates, dendrimers, peptides, or carbohydrates [19].
Liposomal ical rinotecan (nal-IRI, ONIVYDE) was approved in 2015 [20]. Sinchemotherapy still remains the first then, new liposomal irinotecan formulations have been developed and used as second-line treatment of cancer,s of metastatic pancreatic cancer [21][22]. Liposomespecially metastatic tumors are phospholipid bilayers equipped with inner aqueous pockets that are used as drug delivery enhancers of hydrophobic and hydrophilic agents [23]. TuLiposomor drug resistes provide a protective layer that shelters encapsulated drug from the structural alterations or chemical degradation [24]. Furthermore, the covalence and potent adherence of polyethylene glycol (PEG) molecules can be used to improve the systemic circulation of drugs [25]. PEGylated lial side posomal formulation of irinotecan (MM-398) improved the cytotoxic effects are theof irinotecan in a mouse model of brain metastasis compared to irinotecan monotherapy [26]. Liposomal formulatin limiting factors inons of irinotecan are promising new cytotoxic agents that can be utilized to treat other malignancies such as metastatic breast cancer [20]. Zhang et al. [27] conjugancer treatment. These factors promote contted irinotecan with a series of fatty acids to increase its lipophilicity and allow particles to self-assemble in an aqueous environment in order to protect estrified irinotecan from bond hydrolysis by carboxylesterases. This approach resulted in higher intracellular accumulation and an elevated cytotoxicity of irinotecan [27].
One of the mainu factors that favored lipoussomal drug development and research thatformation was the asset of alleviated toxicity due to more directed delivery. Different kinds of carriers have been used to achieve this goal. However, most of them fail to serve their purpose in vivo, while exhibiting a high potential in vitro.
Not only lipostudies tomal formulations but also graphene-based irinotecan formulations were prepared to increase the effects of combined treatments of existing drugsiveness of the drug. Graphene with a sp2-hybridized 2D framework has attracted much attention from scientists because of its outstanding properties. Graphene oxides (GOs) provide a relatively high surface for loading of drug. Moreover, the field of drug delivery is expanding rapidly, raising hope for more efficient anticancer toxidation of graphene results in the formation of chemical groups such as such hydroxyl (–OH), epoxy (> O), and carboxylic (–COOH) groups that can be modified according to the purpose. Furthermore, graphene has high membrane penetrating potential, and the formulations of drugs can be easily uptaken by the cells. Karki et al. [28] performed research on SN-38 loaderapies [1].d on graphene oxides (GOs) modified with Teithese include the use of liposomal formulations, dendrimers, and nanoparticles asr polyvinylpyrrolidone (PVP) or excipient β-cyclodextrin (β-CD). The team analyzed the release of the drug from the nanocarriers and assessed their cytotoxicity in human breast cancer cells (MCF-7). The researchers showed that the SN-38 loaded on nanocarriers exhibited higher cytotoxic potential in the tested cell line. Moreover, the GO–PVP nanocarrier had higher cytotoxic activity than the GO–β-CD nanocarrier, indicating that GO–PVP is a more effective drug delivery systems [28].
Alibolandi et al. A[29] developed a PEGylated acetylated carboxymethylcellulong conventional antineoplastic drugs, several classes are distinguse conjugate of SN38 and covalently attached it to an aptamer against CD133 to ensure the selective delivery of the drug to colorectal cancer stem cells. This approach allowed the nanoparticles to be uptaken by a CD133-expressing HT29 cell line in vitro. Furthermore, the use of nanoconjugates resulted in an enhanced cytotoxicity of the drug compared to the non-targeted self-assembled nanoconjugate [29].
Valencia et al. proposhable: alkyed a combinatory therapy of both cisplatin and irinotecan encapsulated in poly(d,l-lactide-co-glycolide)-co-poly(ethylene g agents, antimetabolites, topoisomerase inhibilycol) (PLGA–PEG)-based nanoparticles. The self-assembly of molecules allowed irinotecan to be passively incorporated into the complexes. The formed nanoparticles were directed toward prostate cancer cells overexpressing prostate-specific membrane antigen (PSMA) receptors, mitotic spindle inhibitors, andby using the PSMA ligand S,S-2-(3-[5-amino-1-carboxypentyl]-ureido)pentanedioic acid. This resulted in selective endocytotic uptake and the controlled release of drug, allowing complexes to act as cytotoxic agents. Both agents exhibited synergistic activities, resulting in elevated cell killing [30].
On the other hand, Onis hi et al. [231][3]. examined irinotecan Tnanopoisomerase I inhibitors have been extensivarticles with poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) for their antitumor potential in mouse sarcoma model in vivo. The group suggested that the tested nanoparticles may exhibit cytotoxic potential in solid tumors distant from the administration site [31].
Machmoudi et al. [32] constructed PEGylated studied since the late 1960s; however, initially, their clinical use was hindered due to their spolyamidoamine (PAMAM) dendrimers containing SN-38 conjugated with peptides: BR2 and CyLoP1. The team assessed both the cytotoxicity and uptake of the formulation by the murine colon carcinoma (C26) cell line. In vitro studies showed that the formulation was much more cytotoxic (range of 154.4-635 nM) compared to SN38 in native form. In addition, the dendrimers were uptaken more promptly by cells compared to SN38. Furthermore, in vivo studies were carried out in order to estimate antitumor efficacy. The results showed that the new formulation enhanced drug accumulation efficiency at the tumor site and exhibited higher anti-tumorigenic efficacy compared to SN-38 alone [32].
Wang et al. developed novel nanopare toxicity and low stabiticles that consisted of hyaluronic acid, poly(lactic-co-glycolic acid), chitosan, and pluronic F-127 as a nanocarrier of irinotecan and doxorubicin [33]. Recently, Hyaluronity.c Acid ChemoTransport (HyACT®) has been employed as a carriage system of Iirinotecan as the first member of this drug group was approved for the. Preclinical studies using the mentioned formulation showed improved responsiveness in CD44 positive tumor cells. In addition, it has been demonstrated that the combination resulted in improved progression-free survival in metastatic colorectal cancer when compared to normal irinotecan monotherapy [34].
Interesting streatudies were carried by Naumann et al. [35]. The team ent of cervical, lung, and ovarian cancer in Japan in 1994mployed SN-38 conjugated to gold nanoparticles via oligonucleotides complementary to specific mRNAs unique to the cancer cells of Ewing sarcoma. In a cancer cell, the SN38-conjugate oligonucleotide is released, allowing the inhibition of topoisomerase by SN-38. The obtained result showed that the drug was efficiently delivered and selectively released in both in vitro and in vivo conditions [35]. I
Zashikhina thet al. [36] developed folself-assembled poly(l-lysine)-b-powly(l-leucinge) (PLys-b-PLeu) years, its use was approved in Europe (1995) and the USA (1996)polymersomes. The cytotoxicity of polypeptide polymerosomes was tested on three cell lines: HEK, NIH-3T3, and A549. The researchers found that the carriers did not exhibit any cytotoxic activity in the tested cell lines. Moreover, the loading of irinotecan into polymersomes resulted in similar antitumor activity in vitro to that observed for free drug [4].
3. Irinotecan (CPT-11)
One of the best-studied topoisomerase I inhibitors is irinotecan. Since its introduction in 1996, 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothecine (CPT-11) has been used to treat various types of cancer such as: pulmonary [5][6][7], pancreatic, gastric [8][9], ovarian [10][11], cervical [12], colorectal [13], and others [14]. Isolated from the Chinese tree Camptotheca acuminata, camptothecin alkaloid was the base for semi-synthetic, water-soluble anologs such as irinotecan (CPT-11, drug name: Camptosar®, Campto®) and topotecan (Hycamtin®) that have been approved by the US Federal Drug Administration for clinical use (in 1996 and 2007 respectively) [15]. Irinotecan is a pentacyclic alkaloid provided with a bis-piperidine side chain, which contributes to its water solubility (Figure 1) [16]. Similar to other campthothecins, irinotecan undergoes structural changes depending on the physiological pH of the cellular environment. Irinotecan is considered to be active in lactone structural form but impermeable through cell membranes and therefore inactive in carboxylate form [17][18].
 Figure 1. Chemical structures of camptothecin and irinotecan.
Figure 1. Chemical structures of camptothecin and irinotecan.
4. New Irinotecan Formulations
Many different approaches were taken to overcome the problem of bioavailability of the drug or its active metabolite SN-38. These include the design of liposomal formulations, nanoparticles, polymer conjugates, dendrimers, peptides, or carbohydrates [19].
Liposomal irinotecan (nal-IRI, ONIVYDE) was approved in 2015 [20]. Since then, new liposomal irinotecan formulations have been developed and used as second-line treatments of metastatic pancreatic cancer [21][22]. Liposomes are phospholipid bilayers equipped with inner aqueous pockets that are used as drug delivery enhancers of hydrophobic and hydrophilic agents [23]. Liposomes provide a protective layer that shelters encapsulated drug from the structural alterations or chemical degradation [24]. Furthermore, the covalent adherence of polyethylene glycol (PEG) molecules can be used to improve the systemic circulation of drugs [25]. PEGylated liposomal formulation of irinotecan (MM-398) improved the cytotoxic effects of irinotecan in a mouse model of brain metastasis compared to irinotecan monotherapy [26]. Liposomal formulations of irinotecan are promising new cytotoxic agents that can be utilized to treat other malignancies such as metastatic breast cancer [20]. Zhang et al. [27] conjugated irinotecan with a series of fatty acids to increase its lipophilicity and allow particles to self-assemble in an aqueous environment in order to protect estrified irinotecan from bond hydrolysis by carboxylesterases. This approach resulted in higher intracellular accumulation and an elevated cytotoxicity of irinotecan [27].
One of the main factors that favored liposomal drug formation was the asset of alleviated toxicity due to more directed delivery. Different kinds of carriers have been used to achieve this goal. However, most of them fail to serve their purpose in vivo, while exhibiting a high potential in vitro.
Not only liposomal formulations but also graphene-based irinotecan formulations were prepared to increase the effectiveness of the drug. Graphene with a sp2-hybridized 2D framework has attracted much attention from scientists because of its outstanding properties. Graphene oxides (GOs) provide a relatively high surface for loading of drug. Moreover, the oxidation of graphene results in the formation of chemical groups such as such hydroxyl (–OH), epoxy (> O), and carboxylic (–COOH) groups that can be modified according to the purpose. Furthermore, graphene has high membrane penetrating potential, and the formulations of drugs can be easily uptaken by the cells. Karki et al. [28] performed research on SN-38 loaded on graphene oxides (GOs) modified with either polyvinylpyrrolidone (PVP) or excipient β-cyclodextrin (β-CD). The team analyzed the release of the drug from the nanocarriers and assessed their cytotoxicity in human breast cancer cells (MCF-7). The researchers showed that the SN-38 loaded on nanocarriers exhibited higher cytotoxic potential in the tested cell line. Moreover, the GO–PVP nanocarrier had higher cytotoxic activity than the GO–β-CD nanocarrier, indicating that GO–PVP is a more effective drug delivery system [28].
Alibolandi et al. [29] developed a PEGylated acetylated carboxymethylcellulose conjugate of SN38 and covalently attached it to an aptamer against CD133 to ensure the selective delivery of the drug to colorectal cancer stem cells. This approach allowed the nanoparticles to be uptaken by a CD133-expressing HT29 cell line in vitro. Furthermore, the use of nanoconjugates resulted in an enhanced cytotoxicity of the drug compared to the non-targeted self-assembled nanoconjugate [29].
Valencia et al. proposed a combinatory therapy of both cisplatin and irinotecan encapsulated in poly(d,l-lactide-co-glycolide)-co-poly(ethylene glycol) (PLGA–PEG)-based nanoparticles. The self-assembly of molecules allowed irinotecan to be passively incorporated into the complexes. The formed nanoparticles were directed toward prostate cancer cells overexpressing prostate-specific membrane antigen (PSMA) receptors, by using the PSMA ligand S,S-2-(3-[5-amino-1-carboxypentyl]-ureido)pentanedioic acid. This resulted in selective endocytotic uptake and the controlled release of drug, allowing complexes to act as cytotoxic agents. Both agents exhibited synergistic activities, resulting in elevated cell killing [30].
On the other hand, Onishi et al. [31] examined irinotecan nanoparticles with poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) for their antitumor potential in mouse sarcoma model in vivo. The group suggested that the tested nanoparticles may exhibit cytotoxic potential in solid tumors distant from the administration site [31].
Machmoudi et al. [32] constructed PEGylated polyamidoamine (PAMAM) dendrimers containing SN-38 conjugated with peptides: BR2 and CyLoP1. The team assessed both the cytotoxicity and uptake of the formulation by the murine colon carcinoma (C26) cell line. In vitro studies showed that the formulation was much more cytotoxic (range of 154.4-635 nM) compared to SN38 in native form. In addition, the dendrimers were uptaken more promptly by cells compared to SN38. Furthermore, in vivo studies were carried out in order to estimate antitumor efficacy. The results showed that the new formulation enhanced drug accumulation efficiency at the tumor site and exhibited higher anti-tumorigenic efficacy compared to SN-38 alone [32].
Wang et al. developed novel nanoparticles that consisted of hyaluronic acid, poly(lactic-co-glycolic acid), chitosan, and pluronic F-127 as a nanocarrier of irinotecan and doxorubicin [33]. Recently, Hyaluronic Acid ChemoTransport (HyACT®) has been employed as a carriage system of irinotecan. Preclinical studies using the mentioned formulation showed improved responsiveness in CD44 positive tumor cells. In addition, it has been demonstrated that the combination resulted in improved progression-free survival in metastatic colorectal cancer when compared to normal irinotecan monotherapy [34].
Interesting studies were carried by Naumann et al. [35]. The team employed SN-38 conjugated to gold nanoparticles via oligonucleotides complementary to specific mRNAs unique to the cancer cells of Ewing sarcoma. In a cancer cell, the SN38-conjugate oligonucleotide is released, allowing the inhibition of topoisomerase by SN-38. The obtained result showed that the drug was efficiently delivered and selectively released in both in vitro and in vivo conditions [35].
Zashikhina et al. [36] developed self-assembled poly(l-lysine)-b-poly(l-leucine) (PLys-b-PLeu) polymersomes. The cytotoxicity of polypeptide polymerosomes was tested on three cell lines: HEK, NIH-3T3, and A549. The researchers found that the carriers did not exhibit any cytotoxic activity in the tested cell lines. Moreover, the loading of irinotecan into polymersomes resulted in similar antitumor activity in vitro to that observed for free drug [36]. The summary of new drug formulations is provided in
. The summary of new drug formulations is provided in
Table 2 below.
below.
Table 2. New irinotecan formulations with effects of modifications and references. New irinotecan formulations with effects of modifications and references.
New Formulation
Effect of Modification
Reference
PEGylated liposomal irinotecan
Improved cytotoxic effects of irinotecan in mouse model of brain metastasis compared to irinotecan monotherapy.
[26]
Irinotecan (Iri)-fatty acid prodrugs (Iri5C, Iri-8C, and Iri-12C) with alkyl chains of different lengths synthesized by esterification using DCC
(dicyclohexylcarbodiimide) and DMAP (4-dimethylamino-pyridine).
Higher intracellular accumulation of the drug and elevated cytotoxicity of irinotecan.
[[27]]
SN-38 loaded on graphene oxides (GOs) modified with either polyvinylpyrrolidone (PVP) or excipient β-cyclodextrin (β-CD).
SN-38 loaded on nanocarriers exhibited higher cytotoxic potential in the MCF-7 cell line. The GO–PVP nanocarrier had higher cytotoxic activity than the GO-β-CD nanocarrier, indicating that the GO–PVP nanocarrier is a more effective drug delivery system.
[[28]]
PEGylated acetylated carboxymethylcellulose conjugate of SN38 covalently attached it to an aptamer against CD133.
Enhanced uptake of the carrier-containing drug by the CD133-expressing HT29 cell line in vitro. The use of nanoconjugates results in an enhanced cytotoxicity of the drug compared to the non-targeted self-assembled nanoconjugate.
[[29]]
Cisplatin and irinotecan encapsulated in poly( d , l -lactide- co -glycolide)- co -poly(ethylene glycol) (PLGA–PEG)-based nanoparticles directed toward prostate cancer cells overexpressing PSMA receptors, by using PSMA ligand-S,S-2-(3-[5-amino-1-carboxypentyl]-ureido)pentanedioic acid.
Selective endocytotic uptake and controlled release of drug, allowing complexes to act as cytotoxic agents. Both agents exhibited synergistic activities, resulting in elevated cell killing.
[[30]]
Nanoparticle system prepared with poly(DL-lactic acid) (PLA), poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEG–PPG–PEG), and irinotecan.
Enhanced antitumor effect against Sarcoma 180 solid tumor. Nanoparticles may exhibit cytotoxic potential in solid tumors, distant from the administration site.
[[31]]
PEGylated polyamidoamine (PAMAM) dendrimers containing SN-38 conjugated with peptides-BR2 and CyLoP1.
Formulation is much more cytotoxic in the murine colon carcinoma (C26) cell line compared to SN38 in its native form. Enhanced uptake of the drug by cells and higher cytotoxicity was observed in vivo for the formulation compared to SN-38 alone.
[[32]]
Hyaluronic Acid ChemoTransport (HyACT ® )
Improved responsiveness in CD44 positive tumor cells. In addition, a combination of improved progression-free survival in metastatic colorectal cancer has been demonstrated, when compared to normal irinotecan monotherapy.
[[34]]
SN-38 conjugated to gold nanoparticles via oligonucleotides complementary to specific mRNAs unique to cancer cells of Ewing sarcoma.
The drug was efficiently delivered and selectively released in both in vitro and in vivo conditions.
[[35]]
Self-assemble poly( l -lysine)-b-poly( l -leucine) (PLys-b-PLeu) polymersomes.
The carriers did not exhibit any cytotoxic activity in tested cell lines (HEK, NIH3T3, and A549). Moreover, the loading of irinotecan into polymersomes resulted in similar antitumor activity in vitro to that observed for free drug.
[[36]]

4. New Irinotecan Formulations
Many different approaches were taken to overcome the problem of bioavailability of the drug or its active metabolite SN-38. These include the design of liposomal formulations, nanoparticles, polymer conjugates, dendrimers, peptides, or carbohydrates [19].
Liposomal irinotecan (nal-IRI, ONIVYDE) was approved in 2015 [20]. Since then, new liposomal irinotecan formulations have been developed and used as second-line treatments of metastatic pancreatic cancer [21][22]. Liposomes are phospholipid bilayers equipped with inner aqueous pockets that are used as drug delivery enhancers of hydrophobic and hydrophilic agents [23]. Liposomes provide a protective layer that shelters encapsulated drug from the structural alterations or chemical degradation [24]. Furthermore, the covalent adherence of polyethylene glycol (PEG) molecules can be used to improve the systemic circulation of drugs [25]. PEGylated liposomal formulation of irinotecan (MM-398) improved the cytotoxic effects of irinotecan in a mouse model of brain metastasis compared to irinotecan monotherapy [26]. Liposomal formulations of irinotecan are promising new cytotoxic agents that can be utilized to treat other malignancies such as metastatic breast cancer [20]. Zhang et al. [27] conjugated irinotecan with a series of fatty acids to increase its lipophilicity and allow particles to self-assemble in an aqueous environment in order to protect estrified irinotecan from bond hydrolysis by carboxylesterases. This approach resulted in higher intracellular accumulation and an elevated cytotoxicity of irinotecan [27].
One of the main factors that favored liposomal drug formation was the asset of alleviated toxicity due to more directed delivery. Different kinds of carriers have been used to achieve this goal. However, most of them fail to serve their purpose in vivo, while exhibiting a high potential in vitro.
Not only liposomal formulations but also graphene-based irinotecan formulations were prepared to increase the effectiveness of the drug. Graphene with a sp2-hybridized 2D framework has attracted much attention from scientists because of its outstanding properties. Graphene oxides (GOs) provide a relatively high surface for loading of drug. Moreover, the oxidation of graphene results in the formation of chemical groups such as such hydroxyl (–OH), epoxy (> O), and carboxylic (–COOH) groups that can be modified according to the purpose. Furthermore, graphene has high membrane penetrating potential, and the formulations of drugs can be easily uptaken by the cells. Karki et al. [28] performed research on SN-38 loaded on graphene oxides (GOs) modified with either polyvinylpyrrolidone (PVP) or excipient β-cyclodextrin (β-CD). The team analyzed the release of the drug from the nanocarriers and assessed their cytotoxicity in human breast cancer cells (MCF-7). The researchers showed that the SN-38 loaded on nanocarriers exhibited higher cytotoxic potential in the tested cell line. Moreover, the GO–PVP nanocarrier had higher cytotoxic activity than the GO–β-CD nanocarrier, indicating that GO–PVP is a more effective drug delivery system [28].
Alibolandi et al. [29] developed a PEGylated acetylated carboxymethylcellulose conjugate of SN38 and covalently attached it to an aptamer against CD133 to ensure the selective delivery of the drug to colorectal cancer stem cells. This approach allowed the nanoparticles to be uptaken by a CD133-expressing HT29 cell line in vitro. Furthermore, the use of nanoconjugates resulted in an enhanced cytotoxicity of the drug compared to the non-targeted self-assembled nanoconjugate [29].
Valencia et al. proposed a combinatory therapy of both cisplatin and irinotecan encapsulated in poly(d,l-lactide-co-glycolide)-co-poly(ethylene glycol) (PLGA–PEG)-based nanoparticles. The self-assembly of molecules allowed irinotecan to be passively incorporated into the complexes. The formed nanoparticles were directed toward prostate cancer cells overexpressing prostate-specific membrane antigen (PSMA) receptors, by using the PSMA ligand S,S-2-(3-[5-amino-1-carboxypentyl]-ureido)pentanedioic acid. This resulted in selective endocytotic uptake and the controlled release of drug, allowing complexes to act as cytotoxic agents. Both agents exhibited synergistic activities, resulting in elevated cell killing [30].
On the other hand, Onishi et al. [31] examined irinotecan nanoparticles with poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) for their antitumor potential in mouse sarcoma model in vivo. The group suggested that the tested nanoparticles may exhibit cytotoxic potential in solid tumors distant from the administration site [31].
Machmoudi et al. [32] constructed PEGylated polyamidoamine (PAMAM) dendrimers containing SN-38 conjugated with peptides: BR2 and CyLoP1. The team assessed both the cytotoxicity and uptake of the formulation by the murine colon carcinoma (C26) cell line. In vitro studies showed that the formulation was much more cytotoxic (range of 154.4-635 nM) compared to SN38 in native form. In addition, the dendrimers were uptaken more promptly by cells compared to SN38. Furthermore, in vivo studies were carried out in order to estimate antitumor efficacy. The results showed that the new formulation enhanced drug accumulation efficiency at the tumor site and exhibited higher anti-tumorigenic efficacy compared to SN-38 alone [32].
Wang et al. developed novel nanoparticles that consisted of hyaluronic acid, poly(lactic-co-glycolic acid), chitosan, and pluronic F-127 as a nanocarrier of irinotecan and doxorubicin [33]. Recently, Hyaluronic Acid ChemoTransport (HyACT®) has been employed as a carriage system of irinotecan. Preclinical studies using the mentioned formulation showed improved responsiveness in CD44 positive tumor cells. In addition, it has been demonstrated that the combination resulted in improved progression-free survival in metastatic colorectal cancer when compared to normal irinotecan monotherapy [34].
Interesting studies were carried by Naumann et al. [35]. The team employed SN-38 conjugated to gold nanoparticles via oligonucleotides complementary to specific mRNAs unique to the cancer cells of Ewing sarcoma. In a cancer cell, the SN38-conjugate oligonucleotide is released, allowing the inhibition of topoisomerase by SN-38. The obtained result showed that the drug was efficiently delivered and selectively released in both in vitro and in vivo conditions [35].
Zashikhina et al. [36] developed self-assembled poly(l-lysine)-b-poly(l-leucine) (PLys-b-PLeu) polymersomes. The cytotoxicity of polypeptide polymerosomes was tested on three cell lines: HEK, NIH-3T3, and A549. The researchers found that the carriers did not exhibit any cytotoxic activity in the tested cell lines. Moreover, the loading of irinotecan into polymersomes resulted in similar antitumor activity in vitro to that observed for free drug [36]. The summary of new drug formulations is provided in
. The summary of new drug formulations is provided in
Table 2 below.
below.
Table 2. New irinotecan formulations with effects of modifications and references. New irinotecan formulations with effects of modifications and references.
New Formulation
Effect of Modification
Reference
PEGylated liposomal irinotecan
Improved cytotoxic effects of irinotecan in mouse model of brain metastasis compared to irinotecan monotherapy.
[26]
Irinotecan (Iri)-fatty acid prodrugs (Iri5C, Iri-8C, and Iri-12C) with alkyl chains of different lengths synthesized by esterification using DCC
(dicyclohexylcarbodiimide) and DMAP (4-dimethylamino-pyridine).
Higher intracellular accumulation of the drug and elevated cytotoxicity of irinotecan.
[[27]]
SN-38 loaded on graphene oxides (GOs) modified with either polyvinylpyrrolidone (PVP) or excipient β-cyclodextrin (β-CD).
SN-38 loaded on nanocarriers exhibited higher cytotoxic potential in the MCF-7 cell line. The GO–PVP nanocarrier had higher cytotoxic activity than the GO-β-CD nanocarrier, indicating that the GO–PVP nanocarrier is a more effective drug delivery system.
[[28]]
PEGylated acetylated carboxymethylcellulose conjugate of SN38 covalently attached it to an aptamer against CD133.
Enhanced uptake of the carrier-containing drug by the CD133-expressing HT29 cell line in vitro. The use of nanoconjugates results in an enhanced cytotoxicity of the drug compared to the non-targeted self-assembled nanoconjugate.
[[29]]
Cisplatin and irinotecan encapsulated in poly( d , l -lactide- co -glycolide)- co -poly(ethylene glycol) (PLGA–PEG)-based nanoparticles directed toward prostate cancer cells overexpressing PSMA receptors, by using PSMA ligand-S,S-2-(3-[5-amino-1-carboxypentyl]-ureido)pentanedioic acid.
Selective endocytotic uptake and controlled release of drug, allowing complexes to act as cytotoxic agents. Both agents exhibited synergistic activities, resulting in elevated cell killing.
[[30]]
Nanoparticle system prepared with poly(DL-lactic acid) (PLA), poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEG–PPG–PEG), and irinotecan.
Enhanced antitumor effect against Sarcoma 180 solid tumor. Nanoparticles may exhibit cytotoxic potential in solid tumors, distant from the administration site.
[[31]]
PEGylated polyamidoamine (PAMAM) dendrimers containing SN-38 conjugated with peptides-BR2 and CyLoP1.
Formulation is much more cytotoxic in the murine colon carcinoma (C26) cell line compared to SN38 in its native form. Enhanced uptake of the drug by cells and higher cytotoxicity was observed in vivo for the formulation compared to SN-38 alone.
[[32]]
Hyaluronic Acid ChemoTransport (HyACT ® )
Improved responsiveness in CD44 positive tumor cells. In addition, a combination of improved progression-free survival in metastatic colorectal cancer has been demonstrated, when compared to normal irinotecan monotherapy.
[[34]]
SN-38 conjugated to gold nanoparticles via oligonucleotides complementary to specific mRNAs unique to cancer cells of Ewing sarcoma.
The drug was efficiently delivered and selectively released in both in vitro and in vivo conditions.
[[35]]
Self-assemble poly( l -lysine)-b-poly( l -leucine) (PLys-b-PLeu) polymersomes.
The carriers did not exhibit any cytotoxic activity in tested cell lines (HEK, NIH3T3, and A549). Moreover, the loading of irinotecan into polymersomes resulted in similar antitumor activity in vitro to that observed for free drug.
[[36]]
New irinotecan formulations with effects of modifications and references.
| New Formulation | Effect of Modification | Reference |
|---|
| PEGylated liposomal irinotecan | Improved cytotoxic effects of irinotecan in mouse model of brain metastasis compared to irinotecan monotherapy. | [26] | ||||||||
| Irinotecan (Iri)-fatty acid prodrugs (Iri5C, Iri-8C, and Iri-12C) with alkyl chains of different lengths synthesized by esterification using DCC (dicyclohexylcarbodiimide) and DMAP (4-dimethylamino-pyridine). |
Higher intracellular accumulation of the drug and elevated cytotoxicity of irinotecan. | [[27]] | ||||||||
| SN-38 loaded on graphene oxides (GOs) modified with either polyvinylpyrrolidone (PVP) or excipient β-cyclodextrin (β-CD). | SN-38 loaded on nanocarriers exhibited higher cytotoxic potential in the MCF-7 cell line. The GO–PVP nanocarrier had higher cytotoxic activity than the GO-β-CD nanocarrier, indicating that the GO–PVP nanocarrier is a more effective drug delivery system. | [[28]] | ||||||||
| PEGylated acetylated carboxymethylcellulose conjugate of SN38 covalently attached it to an aptamer against CD133. | Enhanced uptake of the carrier-containing drug by the CD133-expressing HT29 cell line in vitro. The use of nanoconjugates results in an enhanced cytotoxicity of the drug compared to the non-targeted self-assembled nanoconjugate. | [[29]] | ||||||||
| Cisplatin and irinotecan encapsulated in poly( | d | , | l | -lactide- | co | -glycolide)- | co | -poly(ethylene glycol) (PLGA–PEG)-based nanoparticles directed toward prostate cancer cells overexpressing PSMA receptors, by using PSMA ligand-S,S-2-(3-[5-amino-1-carboxypentyl]-ureido)pentanedioic acid. | Selective endocytotic uptake and controlled release of drug, allowing complexes to act as cytotoxic agents. Both agents exhibited synergistic activities, resulting in elevated cell killing. | [[30]] |
| Nanoparticle system prepared with poly(DL-lactic acid) (PLA), poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEG–PPG–PEG), and irinotecan. | Enhanced antitumor effect against Sarcoma 180 solid tumor. Nanoparticles may exhibit cytotoxic potential in solid tumors, distant from the administration site. | [[31]] | ||||||||
| PEGylated polyamidoamine (PAMAM) dendrimers containing SN-38 conjugated with peptides-BR2 and CyLoP1. | Formulation is much more cytotoxic in the murine colon carcinoma (C26) cell line compared to SN38 in its native form. Enhanced uptake of the drug by cells and higher cytotoxicity was observed in vivo for the formulation compared to SN-38 alone. | [[32]] | ||||||||
| Hyaluronic Acid ChemoTransport (HyACT | ® | ) | Improved responsiveness in CD44 positive tumor cells. In addition, a combination of improved progression-free survival in metastatic colorectal cancer has been demonstrated, when compared to normal irinotecan monotherapy. | [[34]] | ||||||
| SN-38 conjugated to gold nanoparticles via oligonucleotides complementary to specific mRNAs unique to cancer cells of Ewing sarcoma. | The drug was efficiently delivered and selectively released in both in vitro and in vivo conditions. | [[35]] | ||||||||
| Self-assemble poly( | l | -lysine)-b-poly( | l | -leucine) (PLys-b-PLeu) polymersomes. | The carriers did not exhibit any cytotoxic activity in tested cell lines (HEK, NIH3T3, and A549). Moreover, the loading of irinotecan into polymersomes resulted in similar antitumor activity in vitro to that observed for free drug. | [[36]] |
References
- Edgar Pérez-Herrero; Alberto Fernandez-Medarde; Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. European Journal of Pharmaceutics and Biopharmaceutics 2015, 93, 52-79, 10.1016/j.ejpb.2015.03.018.
- Susanne Nussbaumer; Pascal Bonnabry; Jean-Luc Veuthey; Sandrine Fleury-Souverain; Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265-2289, 10.1016/j.talanta.2011.08.034.
- Karol Bukowski; Mateusz Kciuk; Renata Kontek; Mechanisms of Multidrug Resistance in Cancer Chemotherapy.. International Journal of Molecular Sciences 2020, 21, 3233, 10.3390/ijms21093233.
- Emanuela Martino; Serena Della Volpe; Elisa Terribile; Emanuele Benetti; Mirena Sakaj; Adriana Centamore; Andrea Sala; Simona Collina; The long story of camptothecin: From traditional medicine to drugs. Bioorganic & Medicinal Chemistry Letters 2017, 27, 701-707, 10.1016/j.bmcl.2016.12.085.
- Xueqin Yang; Chong-Yi Li; Mingfang Xu; Hong Zhao; Dong Wang; Comparison of first-line chemotherapy based on irinotecan or other drugs to treat non-small cell lung cancer in stage IIIB/IV: a systematic review and meta-analysis.. BMC Cancer 2015, 15, 949, 10.1186/s12885-015-1978-2.
- C J Langer; The emerging world role of irinotecan in lung cancer.. Oncology (Williston Park, N.Y.) 2001, 15, -, -.
- Alan Sandler; Irinotecan plus cisplatin in small-cell lung cancer.. Oncology (Williston Park, N.Y.) 2002, 16, -, -.
- Peter C. Enzinger; Matthew H. Kulke; Jeffrey W. Clark; David P. Ryan; Haesook Kim; Craig C. Earle; Michele M. Vincitore; Ann L. Michelini; Robert J. Mayer; Charles S. Fuchs; et al. A Phase II Trial of Irinotecan in Patients with Previously Untreated Advanced Esophageal and Gastric Adenocarcinoma. Digestive Diseases and Sciences 2005, 50, 2218-2223, 10.1007/s10620-005-3038-2.
- Akitaka Makiyama; Kohei Arimizu; Gen Hirano; Chinatsu Makiyama; Yuzo Matsushita; Tsuyoshi Shirakawa; Hirofumi Ohmura; Masato Komoda; Keita Uchino; Kyoko Inadomi; et al.Shuji AritaHiroshi AriyamaHitoshi KusabaYudai ShinoharaMiyuki KuwayamaTatsuhiro KajitaniHisanobu OdaTaito EsakiKoichi AkashiEishi Baba Irinotecan monotherapy as third-line or later treatment in advanced gastric cancer. Gastric Cancer 2017, 21, 464-472, 10.1007/s10120-017-0759-9.
- David M Gershenson; Irinotecan in epithelial ovarian cancer.. Oncology (Williston Park, N.Y.) 2002, 16, -, -.
- Fernanda Musa; Bhavana Pothuri; Stephanie V. Blank; Huichung T. Ling; James L. Speyer; John Curtin; Leslie Boyd; Xiaochun Li; Judith D. Goldberg; Franco Muggia; et al.Amy Tiersten Phase II study of irinotecan in combination with bevacizumab in recurrent ovarian cancer. Gynecologic Oncology 2017, 144, 279-284, 10.1016/j.ygyno.2016.11.043.
- Claire Verschraegen; Irinotecan for the treatment of cervical cancer.. Oncology (Williston Park, N.Y.) 2002, 16, -, -.
- Charles S. Fuchs; Edith P. Mitchell; Paulo M. Hoff; Irinotecan in the treatment of colorectal cancer. Cancer Treatment Reviews 2006, 32, 491-503, 10.1016/j.ctrv.2006.07.001.
- Mace L. Rothenberg; Irinotecan (CPT‐11): Recent Developments and Future Directions–Colorectal Cancer and Beyond. The Oncologist 2001, 6, 66-80, 10.1634/theoncologist.6-1-66.
- Yves Pommier; Drugging Topoisomerases: Lessons and Challenges. ACS Chemical Biology 2013, 8, 82-95, 10.1021/cb300648v.
- Seigo Sawada; Shun-Ichi Matsuoka; Ken-Ichiro Nokata; Hiroshi Nagata; Tomio Furuta; Teruo Yokokura; Tadashi Miyasaka; Synthesis and Antitumor Activity of 20(S)-Camptothecin Derivatives: A-Ring Modified and 7,10-Disubstituted Camptothecins.. CHEMICAL & PHARMACEUTICAL BULLETIN 1991, 39, 3183-3188, 10.1248/cpb.39.3183.
- Craig J. Thomas; Nicolas J. Rahier; Sidney M. Hecht; Camptothecin: current perspectives. Bioorganic & Medicinal Chemistry 2004, 12, 1585-1604, 10.1016/j.bmc.2003.11.036.
- Yves Pommier; Topoisomerase I inhibitors: camptothecins and beyond. Nature Reviews Cancer 2006, 6, 789-802, 10.1038/nrc1977.
- Xuanrong Sun; Dabu Zhu; Yue Cai; Guobang Shi; Mengshi Gao; Minzi Zheng; One-step mechanochemical preparation and prominent antitumor activity of SN-38 self-micelle solid dispersion.. International Journal of Nanomedicine 2019, 14, 2115-2126, 10.2147/IJN.S193783.
- Nicholas Bernards; Manuela Ventura; Inga B. Fricke; Bart S. Hendriks; Jonathan Fitzgerald; Helen Lee; Jinzi Zheng; Liposomal Irinotecan Achieves Significant Survival and Tumor Burden Control in a Triple Negative Breast Cancer Model of Spontaneous Metastasis. Molecular Pharmaceutics 2018, 15, 4132-4138, 10.1021/acs.molpharmaceut.8b00540.
- Andrea Wang-Gillam; Richard A. Hubner; Jens T. Siveke; Daniel D. Von Hoff; Bruce Belanger; Floris A. De Jong; Beloo Mirakhur; Li-Tzong Chen; NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. European Journal of Cancer 2019, 108, 78-87, 10.1016/j.ejca.2018.12.007.
- Wonhee Woo; Edward T. Carey; Minsig Choi; Spotlight on liposomal irinotecan for metastatic pancreatic cancer: patient selection and perspectives.. OncoTargets and Therapy 2019, 12, 1455-1463, 10.2147/OTT.S167590.
- Gerben A. Koning; Gert Storm; Targeted drug delivery systems for the intracellular delivery of macromolecular drugs.. Drug Discovery Today 2003, 8, 482-3, 10.1016/s1359-6446(03)02699-0.
- Chan Wu; Yang Zhang; Daoqiu Yang; Jinfeng Zhang; Juanjuan Ma; Dan Cheng; Jianming Chen; Li Deng; Novel SN38 derivative-based liposome as anticancer prodrug: an in vitro and in vivo study. International Journal of Nanomedicine 2018, 14, 75-85, 10.2147/IJN.S187906.
- J. Milton Harris; Robert B. Chess; Effect of pegylation on pharmaceuticals. Nature Reviews Drug Discovery 2003, 2, 214-221, 10.1038/nrd1033.
- Afroz S. Mohammad; Jessica I. Griffith; Chris E. Adkins; Neal Shah; Emily Sechrest; Emma L. Dolan; Tori B. Terrell-Hall; Bart S. Hendriks; Helen Lee; Paul R. Lockman; et al. Liposomal Irinotecan Accumulates in Metastatic Lesions, Crosses the Blood-Tumor Barrier (BTB), and Prolongs Survival in an Experimental Model of Brain Metastases of Triple Negative Breast Cancer. Pharmaceutical Research 2018, 35, 31-31, 10.1007/s11095-017-2278-0.
- Chunqiu Zhang; Shubin Jin; Xiangdong Xue; Tingbin Zhang; Yonggang Jiang; Paul C. Wang; Xing-Jie Liang; Tunable self-assembly of Irinotecan-fatty acid prodrugs with increased cytotoxicity to cancer cells†. Journal of Materials Chemistry B 2016, 4, 3286-3291, 10.1039/c6tb00612d.
- Neha Karki; Himani Tiwari; Mintu Pal; Alok Chaurasia; Rajaram Bal; Penny Joshi; Nanda Gopal Sahoo; Functionalized graphene oxides for drug loading, release and delivery of poorly water soluble anticancer drug: A comparative study. Colloids and Surfaces B: Biointerfaces 2018, 169, 265-272, 10.1016/j.colsurfb.2018.05.022.
- Mona Alibolandi; Khalil Abnous; Sajjad Anvari; Marzieh Mohammadi; Mohammad Ramezani; Seyed Mohammad Taghdisi; CD133-targeted delivery of self-assembled PEGylated carboxymethylcellulose-SN38 nanoparticles to colorectal cancer. Artificial Cells, Nanomedicine, and Biotechnology 2018, 46, 1159-1169, 10.1080/21691401.2018.1446969.
- Pedro M. Valencia; Eric M. Pridgen; Brian Perea; Suresh Gadde; Christopher Sweeney; Philip W. Kantoff; Neil H Bander; Stephen J. Lippard; Robert Langer; Rohit Karnik; et al.Omid C. Farokhzad Synergistic cytotoxicity of irinotecan and cisplatin in dual-drug targeted polymeric nanoparticles. Nanomedicine 2013, 8, 687-698, 10.2217/nnm.12.134.
- Hiraku Onishi; Yoshiaki Machida; Yoshiharu Machida; Antitumor properties of irinotecan-containing nanoparticles prepared using poly(DL-lactic acid) and poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol).. Biological & Pharmaceutical Bulletin 2003, 26, 116-119, 10.1248/bpb.26.116.
- Asma Mahmoudi; Mahmoud Reza Jaafari; Navid Ramezanian; Leila Gholami; Bizhan Malaekeh-Nikouei; BR2 and CyLoP1 enhance in-vivo SN38 delivery using pegylated PAMAM dendrimers.. International Journal of Pharmaceutics 2019, 564, 77-89, 10.1016/j.ijpharm.2019.04.037.
- Hai Wang; Pranay Agarwal; Shuting Zhao; Ronald X. Xu; Jianhua Yu; Xiongbin Lu; XiaoMing He; Hyaluronic acid-decorated dual responsive nanoparticles of Pluronic F127, PLGA, and chitosan for targeted co-delivery of doxorubicin and irinotecan to eliminate cancer stem-like cells.. Biomaterials 2015, 72, 74-89, 10.1016/j.biomaterials.2015.08.048.
- Muhammad Alamgeer; D. Neil Watkins; Ilia Banakh; Beena Kumar; Ben Markman; Vinod Ganju; Daniel Gough; A phase IIa study of HA-irinotecan, formulation of hyaluronic acid and irinotecan targeting CD44 in extensive-stage small cell lung cancer. Investigational New Drugs 2017, 36, 288-298, 10.1007/s10637-017-0555-8.
- Jordan A. Naumann; John C. Widen; Leslie A. Jonart; Maryam Ebadi; Jian Tang; David J Gordon; Daniel A. Harki; Peter M. Gordon; SN-38 Conjugated Gold Nanoparticles Activated by Ewing Sarcoma Specific mRNAs Exhibit In Vitro and In Vivo Efficacy. Bioconjugate Chemistry 2018, 29, 1111-1118, 10.1021/acs.bioconjchem.7b00774.
- N.N. Zashikhina; M.V. Volokitina; V.A. Korzhikov-Vlakh; I.I. Tarasenko; A. Lavrentieva; T. Scheper; E. Rühl; R.V. Orlova; Tatiana B. Tennikova; E.G. Korzhikova-Vlakh; et al. Self-assembled polypeptide nanoparticles for intracellular irinotecan delivery. European Journal of Pharmaceutical Sciences 2017, 109, 1-12, 10.1016/j.ejps.2017.07.022.
